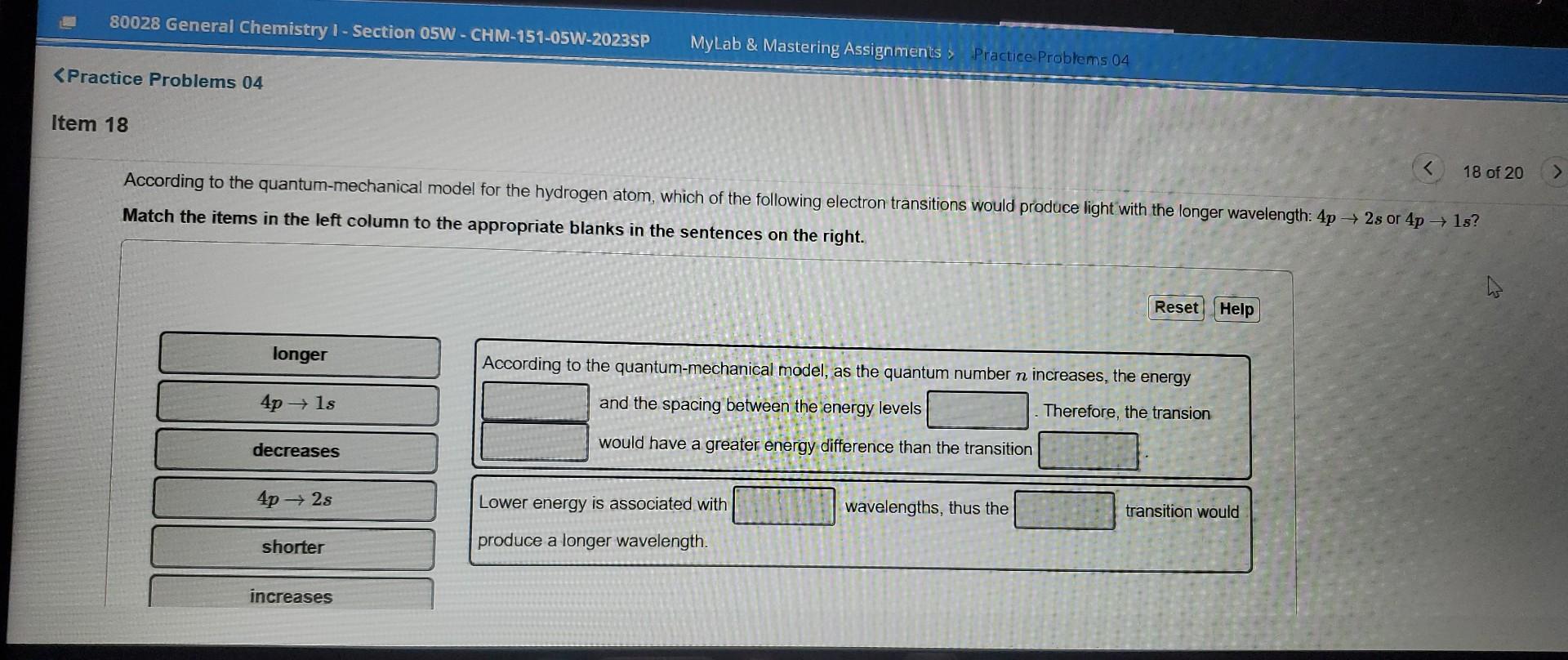
Question: please answer asap According to the quantum-mechanical model for the hydrogen atom, which of the following electron transitions would produce light with the longer wavelength:
please answer asap
According to the quantum-mechanical model for the hydrogen atom, which of the following electron transitions would produce light with the longer wavelength: 4p2s or 4p1s? Match the items in the left column to the appropriate blanks in the sentences on the right. According to the quantum-mechanical model, as the quantum number n increases, the energy and the spacing between the energy levels Therefore, the transion would have a greater energy difference than the transition Lower energy is associated with wavelengths, thus the produce a longer wavelength
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


