Question: please answer ASAP Question 6 (25 marks) The compressibility factor (Z) is a dimensionless quantity which could be expressed in terms of the molar volume
please answer ASAP
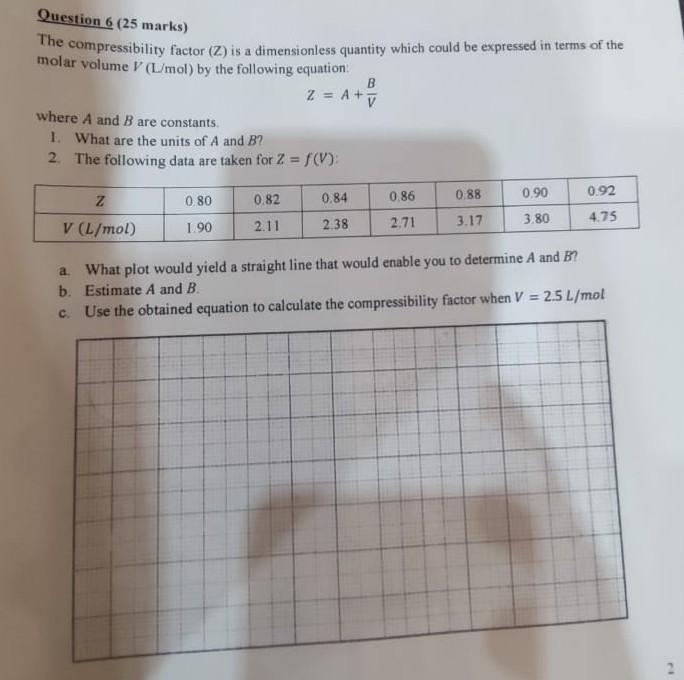
Question 6 (25 marks) The compressibility factor (Z) is a dimensionless quantity which could be expressed in terms of the molar volume V (L/mol) by the following equation B Z = A + ty where A and B are constants. 1. What are the units of A and B? 2. The following data are taken for 2 = f(V): 0.80 0.82 0.84 0.86 0.90 0.92 V (L/mol) 1.90 2.11 2.38 2.71 3.17 3.80 4.75 0.88 Z a What plot would yield a straight line that would enable you to determine A and B? b. Estimate A and B. c. Use the obtained equation to calculate the compressibility factor when V = 2.5 L/mol 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


