Question: q1 q2 The final answer is only I am taking the exam now 7 How many phosphate ions are there in 465 mg of Cas(PO)
q1
q2 The final answer is only I am taking the exam now
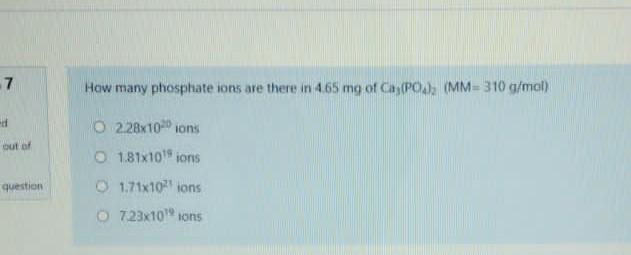
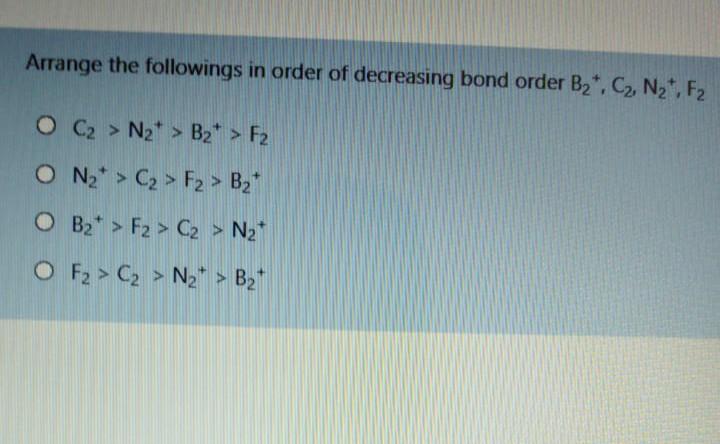
7 How many phosphate ions are there in 465 mg of Cas(PO) (MM = 310 g/mol) d 0 2.28x10 ions but of question 181x10 ions 1.71x10 tons 7.23x10 ons Arrange the followings in order of decreasing bond order B2, C2, N2, F2 O C2 > N2* > B2* > F2 O N2* > C2 > F2 > B2 O B2+ > F2 > C2 > N2 O F2 > C2 > Na+ > Bz+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


