Question: PLEASE ANSWER based on this graph answer the following questions a = 1.3900 Volume vs. Pressure 700 van der Waals equation Ideal Gas equation 600
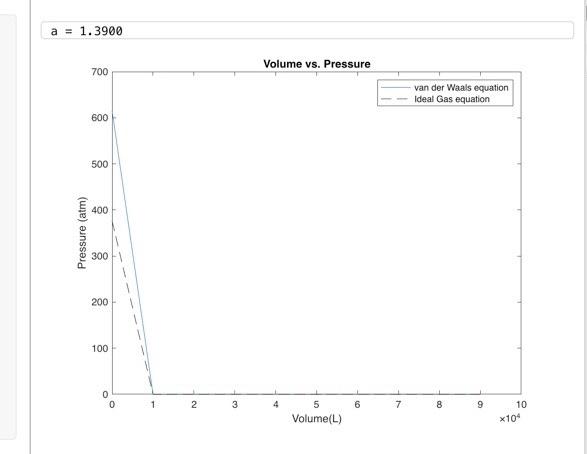

a = 1.3900 Volume vs. Pressure 700 van der Waals equation Ideal Gas equation 600 500 - 400 (atm) Pressure 300 200 100 0 0 1 2 3 4 6 7 7 8 9 5 Volume(L) 10 x10" Q. For which ranges of pressures are the volumes predicted by the real and ideal gas equations almost the same? b. For which ranges of pressures are the volumes predicted by the real and ideal gas equations different? C. Why do the real and ideal gas equations diverge in the range of pressures for which you observed differences
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


