Question: please answer both - Data Rejection. (No separate data set needed, show full work by hand) The following series of masses are taken for an

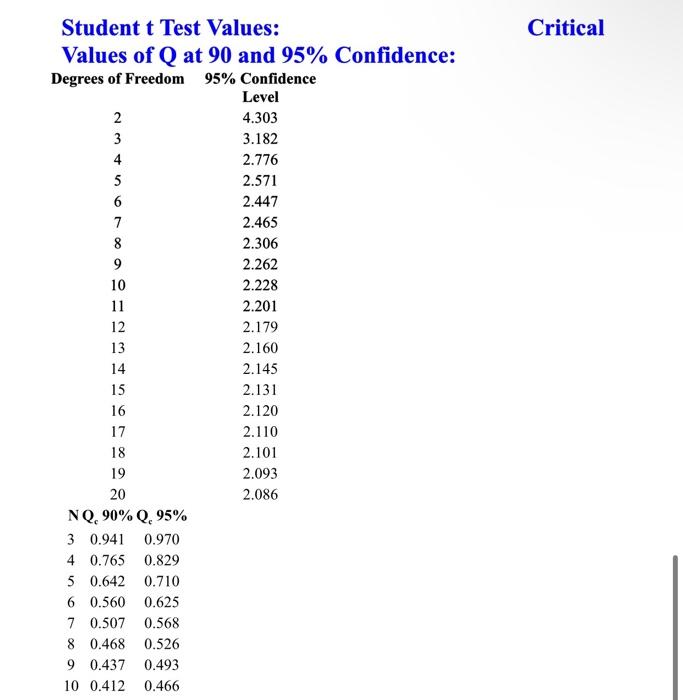
- Data Rejection. (No separate data set needed, show full work by hand) The following series of masses are taken for an unknown sample: 0.134g,0.120g,0.109g,0.128g,0.131g,0.119g,0.136g,0.132g. Determine whether any measurements should be rejected at the 90 percent confidence level, clearly showing work and noting which data points. After rejecting values where appropriate, determine the estimated average and standard deviations, and the 95 percent confidence limit of error. - Propagation of Error. (No separate data set needed, show full work by hand) A quantity Y calculated from three independently measured quantities x,y,z can be determined by Y=f(x,y,z) where x,y, and z have standard uncertainties ux,uy, and ux. As such, determine the number of moles, with standard combined uncertainty included, of an ideal gas from independent measurements of its pressure, volume, and temperature, assuming R=0.082056 L atm /(mol K): P=0.2680.012atmV=1.260.05LT=294.20.3K Student t Test Values: Critical Values of Q at 90 and 95% Confidence: ' Confidence
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


