Question: Please answer both!!! Question 8 0.2 pts 2 Na (s) + Cl2 (g) --> 2 NaCl (s) AH = -822 kJ/mol Calculate the quantity of
Please answer both!!!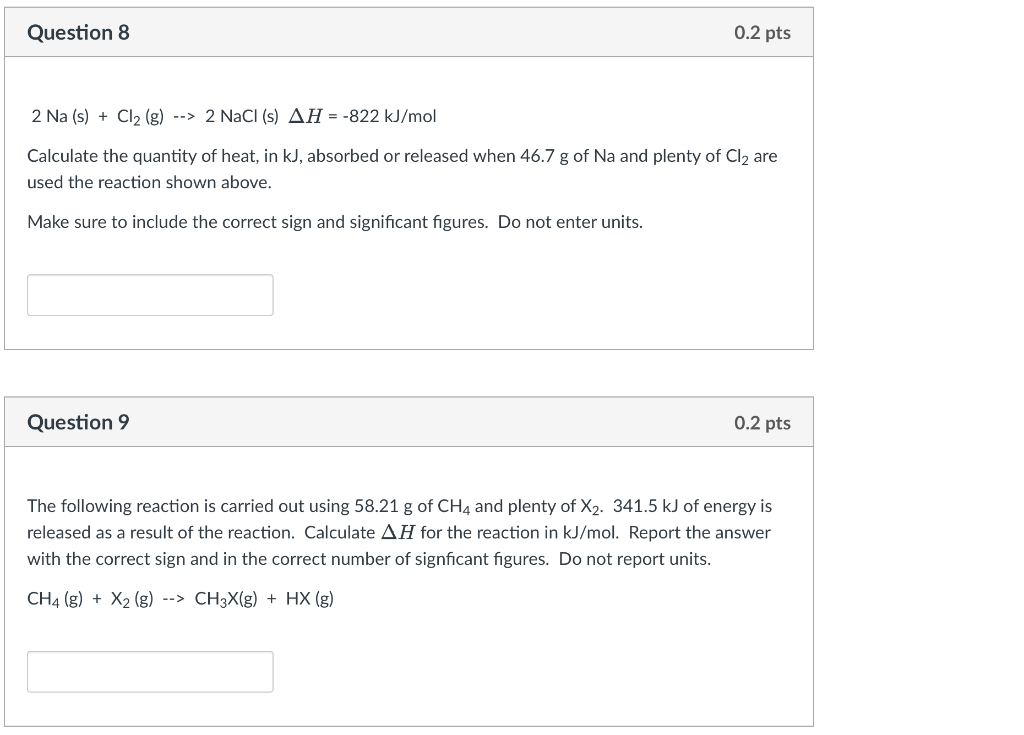
Question 8 0.2 pts 2 Na (s) + Cl2 (g) --> 2 NaCl (s) AH = -822 kJ/mol Calculate the quantity of heat, in kJ, absorbed or released when 46.7 g of Na and plenty of Cl2 are used the reaction shown above. Make sure to include the correct sign and significant figures. Do not enter units. Question 9 0.2 pts The following reaction is carried out using 58.21 g of CH4 and plenty of X2. 341.5 kJ of energy is released as a result of the reaction. Calculate AH for the reaction in kJ/mol. Report the answer with the correct sign and in the correct number of signficant figures. Do not report units. CH4 (g) + X2 (g) --> CH3X(g) + HX (g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


