Question: hello, can I please have help on A,B,C? I attached all needed material. WORKED EXAMPLE 9.3 Calculating the Amount of Heat Released in a Reaction


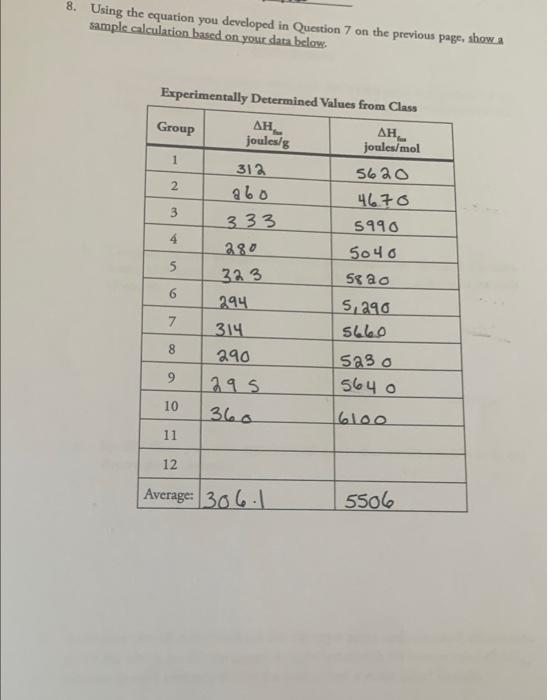

WORKED EXAMPLE 9.3 Calculating the Amount of Heat Released in a Reaction daties in robot when 5.00 gol pomers with sos! 30,00) 4 --2044 IDENTIFY STRATEGY According to the balanced equation, 2044 of het is nied from the reaction of molt program. To find out how much beat is led from the reaction of 5.00 popane, webne to convert from grams to me and the thermochemical corelate quantity to heat SOLUTION The most popu (CH) 44.09 gol.00 of qualis 0.113 mok CH 5.OKCH -0.114 CR CH Themelore 2011 at 0.1154 Cres 23 for heat 2014 0.1114 mol CH Imol CH, CHECK Since the molam od CH is who 4 hal, Hochly 0.1 mol Therefore, the bear volved in touch that the most released tort mol CH. and the ima i nawet of 204 k. which is really done to 232 PRACTICES Use the following thermochemicals to calculate how much heatin kille i evolved or absorbed when 10.0 of water converted to hydrogen and oxygen 2 Hill - 0)HO AH-5716 APPLY 9.6 Approximately 18 x 10 of menys required to how an home in the Midwest during the month of January. Howach propane) must be burned to provide 1.8 X 10k of con CH) + 30,8) 300,00) + 4H0 - 2044 9.6 ENTHALPIES OF CHEMICAL AND PHYSICAL CHANGES Almost every change in a system involves either again or a loss of enthalpy. The change can be either physical, such as the melting of a world to a liquid, or chemical, such as the humning of propane. Let's look at amples of both kinds Enthalpies of Chemical Change Enthalpy change is often called about of reaction because it is a measure of the heat flow into or out of a system at constant pressure. If the products of a reaction have 3 more enthalpy than the reactants, then heat has lowed into the system from the sur roundings and SH has a positive sign. Reactions in which the system gains heat are said to be endothermic fondo means within," so heat flows in. The reaction of 1 mol huwi ding & Chemical and the of barium hydroxide octahydrate with ammonium chloride, for example, absorbs 80.3 from the surroundings (+80,3]). The surroundings, having lost heat. become cold cold, in fact, that the temperature drops below freezing FIGURE 37). Ba(OH), -8 H.O() + 2NH.) BaCl() + 2NHC) - 10H00 H = +80.31 If the products of a reaction have less enthalpy than the actants, then heat has flowed out of them to the surroundings and has a negative sign. Reactions that lose heat to the surroundings are and to be exothermic exo mean out" heat flows out. The thermite reaction of aluminum with iron) side, for instance, releases so much heat (AH - - 52 kJ), and the surroundings get so hot, that the reaction is used in construction work to weld iron. 2. A) - Fest.) --- Fels) + A1:00) AH-852 As noted previously, the value of a given for an equation assumes that the equation is balanced to represent the numbers of moles of reacties and products, that all substances are in their standard states, and that the physical stof each substance is a specified FIGURE 9,7 The endothermic reactio hydride octahydratew chloride. The reaction de heat from the surroundi temperature falls below a Out tity them and the of the end Enthalpies of Physical Change What would happen if you started with a block ofice at a low temperature, say -10C and slowly increased its enthalpy by adding heat? The initial input of heat would cause the temperature of the ice to rise until it reached OC Additional hat would then cause the ice to melt without raising temperature as the added cry expended in overcoming the intermolecular forces that hold H O molecules together in the ice crystal. The amount of heat necessary to me a substance without changing its temperature is called the enthalpy of furon, or heat of AH..). For H.O, SHE = 6.01 kJ/mol at 0 C and the process is endothermic because heat is absorbed. The thermochemical equation representing the heat of fusios of -ONE H0(1) - H.O(1) SHL-601 Once the ice has melted, further input of heat raises the temperature of the liquide until it reaches 100 "Cand adding still more heat the cases the water boil. Once again, energy is necessary to overcome the intermolecular forces holding molecules together in the liquid, so the temperature does not rise til all the liquid has been converted into vapor. The amount of heat required to vaporine a substance without changin temperature is called the exclupy of suporisation, or beat options For H.O.SH-40.7 k/mol at 100, and the process is endother. The ther mochemical equation representing the best of sportation of water HO(I) ---HO) a = 40 k/mol Another kind of physical change in addition to melting and built the direct conversion of a solid to a vapor without going through a liquid Sold CO, deyici), for example, changes directly from solid to aportamospheric without first melting to liquid. Since enthalpy is a state function, the enthalpy change going from solid to vapor must be constant gardless of the path taken. Thus temperature, a substance's talpy of womation, or beat of equals the sum of the heat of fusion and the heat of vaporation (FIGURES Barum deside the compound the roles clustered around the huruma 8. Using the equation you developed in Question 7 on the previous page, showa sample calculation based on your data below. Experimentally Determined Values from Class Group , joules/g 1 2 3 3 . joules/mol 5620 46.70 5990 5040 Se ao 5,290 4 5 312 abo 333 280 323 294 314 290 295 360 6 8 523o 9 5640 10 6100 11 12 Average: 306-1 5506 1. a. Compare your own group's value with the known value (see Section 9.6 in Robinson, McMurry & Fay, 7th or 9th Ed.) of AH, (expressed in joules per mole of water). b. Compare the class average value to the known value (see Section 9.6 in Rob- inson, McMurry & Fay, 7th or 8th Ed.) of AH (expressed in joules per mole of water). fus c. Specify three potential experimental errors that would contribute to any discrepancies observed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


