Question: please answer both questions EXAMPLE PROBLEM 4.4 Composition Conversion-From Weight Percent to Atom Percent Determine the composition. In atom percent of an alloy that consists
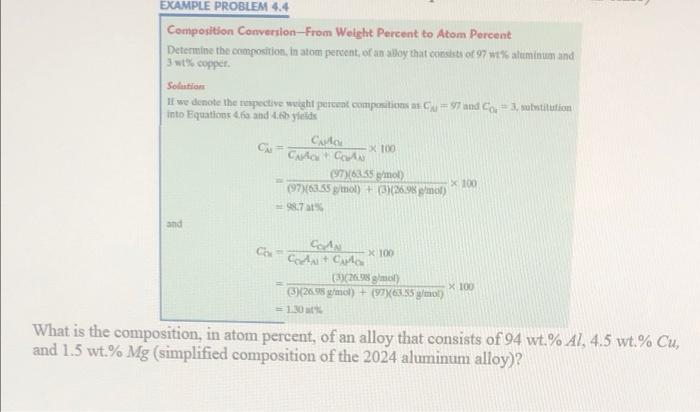

EXAMPLE PROBLEM 4.4 Composition Conversion-From Weight Percent to Atom Percent Determine the composition. In atom percent of an alloy that consists of 97 wt% aluminum and 3 we copper Solution If we donote the respective weight percent compositions as = $7 and Co + 3 wubstitution into Equations to and 4.6 yields Captor = x 100 (7)(8355 mol X 100 (97)(63.35 g/mol) + (326,98 mot) =9871 and Cola Coda Cala (3)(26.998 gol) x 100 3)26.95 gmol) + (97783.55 g/mol) = 130 What is the composition, in atom percent, of an alloy that consists of 94 wt.% A1, 4.5 wt% Cu, and 1.5 wt% Mg (simplified composition of the 2024 aluminum alloy)? x 100 Part D: Determine the ASTM grain size number if 25 grains per square inch are observed at a magnification of 200 X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


