Question: please answer C and E i will upvote. thank you. that (lowerior higher?) melting solid solutions crystallize and freeze out first as the melt cools.
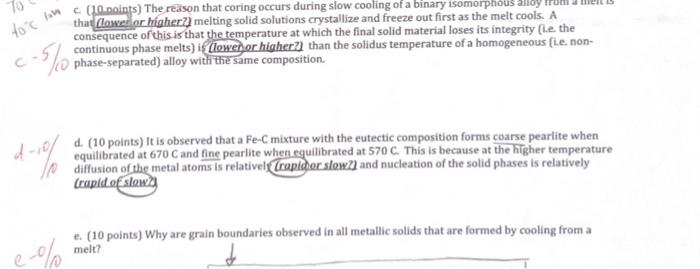
that (lowerior higher?) melting solid solutions crystallize and freeze out first as the melt cools. A consequence of this is that the temperature at which the final solid material loses its integrity (Le the continuous phase melts) i\& (loweh or higher?] than the solidus temperature of a homogeneous (i.e. non- (0) phase-separated) alloy with the same composition. d. (10 points) It is observed that a Fe-C mixture with the eutectic composition forms coarse pearlite when equilibrated at 670C and fine pearlite when equilibrated at 570C. This is because at the higher temperature diffusion of the metal atoms is relativel (rapidor slowz) and nucleation of the solid phases is relatively crapid of slowey
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


