Question: Please answer correctly for great rating Compare the bolling points of the various isomeric hydrocarbons shown in the table below. Notice the relationship between boiling

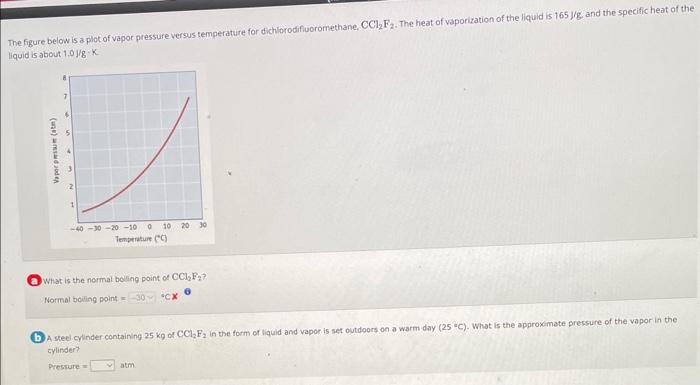
Compare the bolling points of the various isomeric hydrocarbons shown in the table below. Notice the relationship between boiling point and structure; branched-chain hydrocarbons have lower bolling points than the unbranched isomer. Speculate on possible reasons for this trend. Why might the intermolecular forces be slightly different in these compounds? The more branched structure has larger exposed surface and hence stronger dispersion intermolecular forces. The more branched structure has smalier exposed surface and hence weaker dispersion intermolecular forces. The more branched structure has umaler exposed surface and hence stronger dispersion intermolecular forces. The more branched structure has larger exposed surface and hence weaker dispersion intermolecular forces. The figure below is a plot of vapor pressure versus temperature for dichlorodifuoromethane, CCl2F2. The heat of vaporization of the liquid is 165 jgg and the speeific heat of the liquid is about 1.0J/gK W What is the normal boling point of CCl2F2 ? Normalboilingpoint=cCX A steel cylinder containing 25kg of CCl2F2 in the form of liquid and vapor is set outdoors on a warm day (25 " C ). What is the approxemate pressure of the vapor in the cylindert? Presture=atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


