Question: please answer d e and f as brief as possible The particulate model shown below represents the moles of particles present in the container after
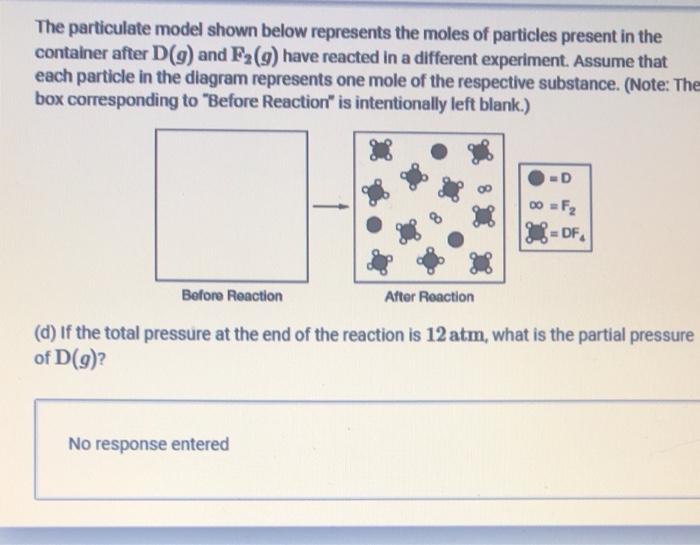

The particulate model shown below represents the moles of particles present in the container after D(9) and F (9) have reacted in a different experiment. Assume that each particle in the diagram represents one mole of the respective substance. (Note: The box corresponding to "Before Reaction" is intentionally left blank.) -D =F2 32 DF Before Reaction After Reaction (d) If the total pressure at the end of the reaction is 12 atm, what is the partial pressure of D(9) No response entered (e) Assuming that only D and Fy are present in the container before the reaction, determine the number of moles of D and F2 that were present in the initial reaction mixture. D= F2 No response entered The equation and enthalpy change for the reaction between D(9) and F2(g) are given below. D(9) +2F2(g) DF (9) AH = -250 kJ/molen () If 0.50 mol of DF4(9) is produced when equimolar amounts of D(9) and F2(g) react, how much heat is released
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


