Question: please answer d e f g and h asap The particulate model shown below represents the moles of particles present in the container after D(g)
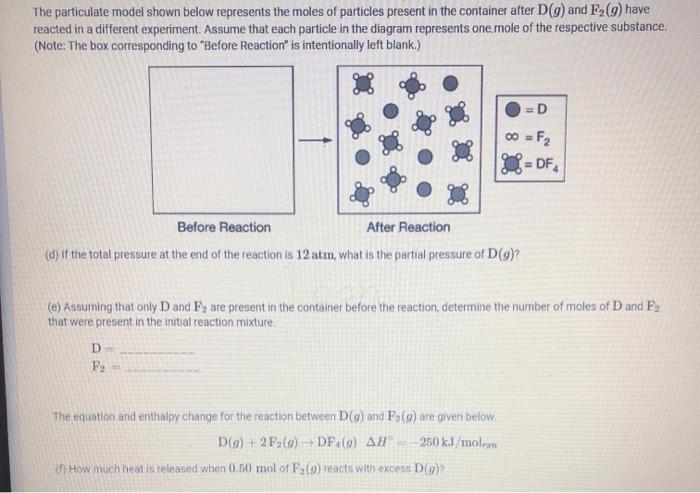
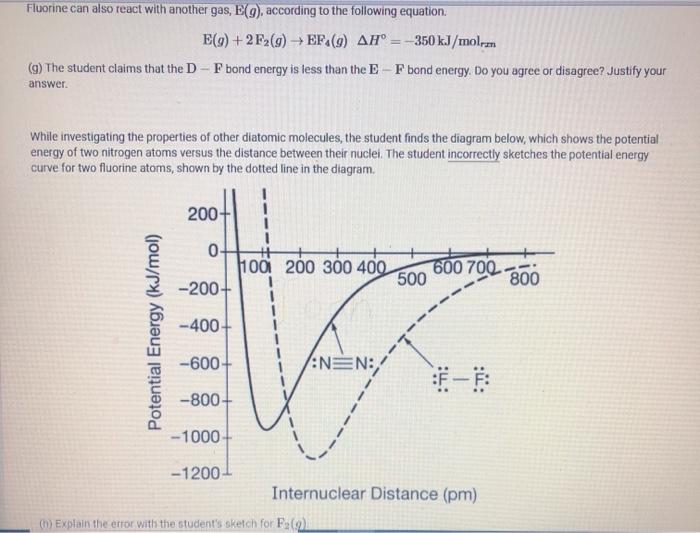
The particulate model shown below represents the moles of particles present in the container after D(g) and F2 (9) have reacted in a different experiment. Assume that each particle in the diagram represents one mole of the respective substance. (Note: The box corresponding to "Before Reaction" is intentionally left blank.) =D 00 = F2 DF Before Reaction After Reaction (d) if the total pressure at the end of the reaction is 12 atm, what is the partial pressure of Dg)? (e) Assuming that only D and Fy are present in the container before the reaction, determine the number of moles of D and F, that were present in the initial reaction mixture D F The equation and enthalpy change for the reaction between DC) and Fs(o) are given below D(9)+2F26) - DF(0) AH 250 kJ/mol How much heat is released when 0.50 mol of F (9) reacts with excess Day Fluorine can also react with another gas, E(9) according to the following equation E(9) +2F2(g) EF (9) AH = -350 kJ/molem (9) The student claims that the D-F bond energy is less than the E-F bond energy. Do you agree or disagree? Justify your answer. While investigating the properties of other diatomic molecules, the student finds the diagram below, which shows the potential energy of two nitrogen atoms versus the distance between their nuclei. The student incorrectly sketches the potential energy curve for two fluorine atoms, shown by the dotted line in the diagram. 200 0 100 200 300 400 . -200+ 600 700 - 500 800 Potential Energy (kJ/mol) -400 -600+ :NEN: -800+ -1000 -1200- Internuclear Distance (pm) () Explain the error with the student's sketch for F (2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


