Question: please answer d, e, f parts of the given problem P12-8A The gas-phase reversible reaction as discussed in P11-ZB is now carried out under high
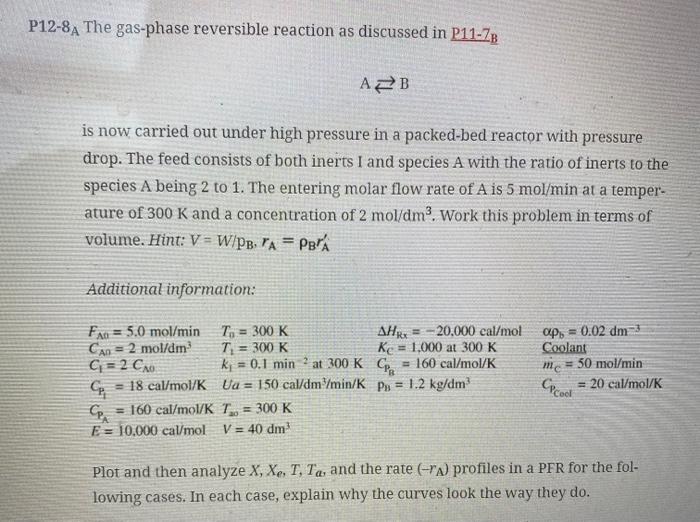

P12-8A The gas-phase reversible reaction as discussed in P11-ZB is now carried out under high pressure in a packed-bed reactor with pressure drop. The feed consists of both inerts I and species A with the ratio of inerts to the species A being 2 to 1. The entering molar flow rate of A is 5 mol/min at a temper- ature of 300 K and a concentration of 2 mol/dm3. Work this problem in terms of volume. Hint: V = W/PB. TA = PBA Additional information: at 300 K CP FA0 = 5.0 mol/min T. = 300 K AH = -20.000 cal/mol Can = 2 mol/dm T = 300 K Ke = 1,000 at 300 K C=2 CAO. k = 0.1 min = 160 cal/mol/K G = 18 cal/mol/K Ua = 150 cal/dm/min/K Pu = 1.2 kg/dm Cp = 160 cal/mol/K T.. = 300 K E = 10,000 cal/mol V = 40 dm aps = 0.02 dm3 Coolant m = 50 mol/min = 20 cal/mol/K Plot and then analyze X, Xe, T, Ta, and the rate (-") profiles in a PFR for the fol- lowing cases. In each case, explain why the curves look the way they do. (d) Compare and contrast each of the above results and the results for adiabatic op- eration (e.g., make a plot or a table of X and Xe obtained in each case). (e) Vary some of the parameters, e.g., (0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


