Question: The value of Kp for the reaction 2H2 S(9) + 2H2(9) + S2() is 1.2 x 10 2 at 1065C. The value of Kc
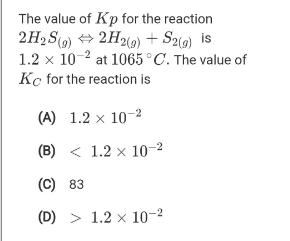
The value of Kp for the reaction 2H2 S(9) + 2H2(9) + S2() is 1.2 x 10 2 at 1065C. The value of Kc for the reaction is (A) 1.2 x 10-2 (B) < 1.2 x 10-2 (C) 83 (D) > 1.2 x 10-2
Step by Step Solution
3.30 Rating (144 Votes )
There are 3 Steps involved in it
Ans The given reaction is 2H2Sg 2H2g S2g Given Kp 12 102 T 1065C 27315 1065 K 133815 K Th... View full answer

Get step-by-step solutions from verified subject matter experts


