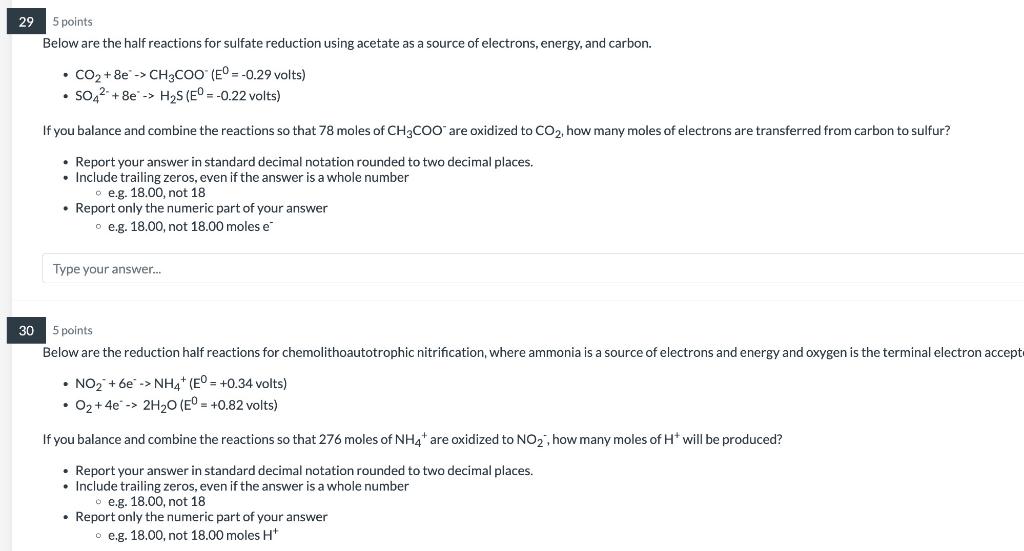
Question: please answer!!!! I will rate it! 95 points Below are the half reactions for sulfate reduction using acetate as a source of electrons, energy, and
please answer!!!! I will rate it!
95 points Below are the half reactions for sulfate reduction using acetate as a source of electrons, energy, and carbon. - CO2+8eCH3COO(E0=0.29 volts ) - SO42+8eH2S(E0=0.22 volts) If you balance and combine the reactions so that 78 moles of CH3COOare oxidized to CO2, how many moles of electrons are transferred from carbon to sulfur? - Report your answer in standard decimal notation rounded to two decimal places. - Include trailing zeros, even if the answer is a whole number - e.g. 18.00, not 18 - Report only the numeric part of your answer - e.g. 18.00, not 18.00 moles e Type your answer... 5 points Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron accep - NO2+6eNH4+(E0=+0.34 volts ) - O2+4e2H2O(E0=+0.82 volts ) If you balance and combine the reactions so that 276 moles of NH4+are oxidized to NO2, how many moles of H+will be produced? - Report your answer in standard decimal notation rounded to two decimal places. - Include trailing zeros, even if the answer is a whole number o.g. 18.00, not 18 - Report only the numeric part of your answer a e.g. 18.00, not 18.00 moles H+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


