Question: both please will rate Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is
both please will rate
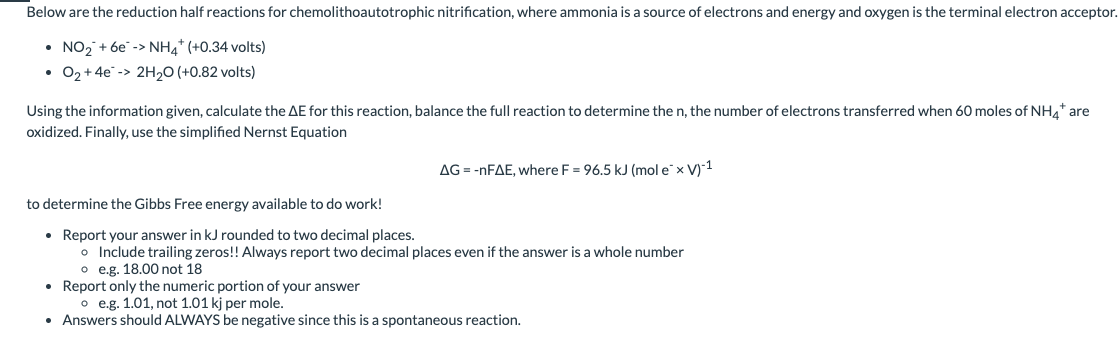
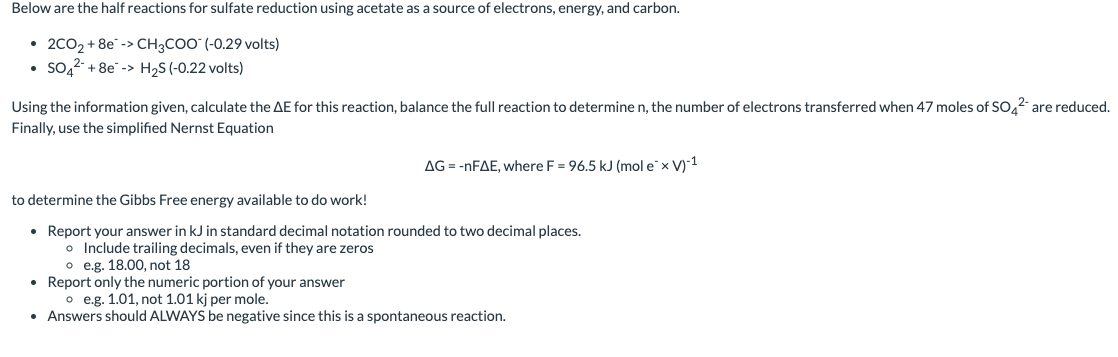
Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron acceptor - NO2+6e>NH4+(+0.34 volts) - O2+4e2H2O (+0.82 volts) Using the information given, calculate the E for this reaction, balance the full reaction to determine the n, the number of electrons transferred when 60 moles of NH4+are oxidized. Finally, use the simplified Nernst Equation G=nFE,whereF=96.5kJ(moleV)1 to determine the Gibbs Free energy available to do work! - Report your answer in kJ rounded to two decimal places. - Include trailing zeros!! Always report two decimal places even if the answer is a whole number - e.g. 18.00 not 18 - Report only the numeric portion of your answer - e.g. 1.01, not 1.01kj per mole. - Answers should ALWAYS be negative since this is a spontaneous reaction. Below are the half reactions for sulfate reduction using acetate as a source of electrons, energy, and carbon. - 2CO2+8eCH3COO(0.29 volts) - SO42+8eH2S(0.22 volts) Using the information given, calculate the E for this reaction, balance the full reaction to determine n, the number of electrons transferred when 47 moles of SO42 are reduced. Finally, use the simplified Nernst Equation G=nFE,whereF=96.5kJ(moleV)1 to determine the Gibbs Free energy available to do work! - Report your answer in kJ in standard decimal notation rounded to two decimal places. - Include trailing decimals, even if they are zeros - e.g. 18.00, not 18 - Report only the numeric portion of your answer - e.g. 1.01, not 1.01kj per mole. - Answers should ALWAYS be negative since this is a spontaneous reaction. Below are the reduction half reactions for chemolithoautotrophic nitrification, where ammonia is a source of electrons and energy and oxygen is the terminal electron acceptor - NO2+6e>NH4+(+0.34 volts) - O2+4e2H2O (+0.82 volts) Using the information given, calculate the E for this reaction, balance the full reaction to determine the n, the number of electrons transferred when 60 moles of NH4+are oxidized. Finally, use the simplified Nernst Equation G=nFE,whereF=96.5kJ(moleV)1 to determine the Gibbs Free energy available to do work! - Report your answer in kJ rounded to two decimal places. - Include trailing zeros!! Always report two decimal places even if the answer is a whole number - e.g. 18.00 not 18 - Report only the numeric portion of your answer - e.g. 1.01, not 1.01kj per mole. - Answers should ALWAYS be negative since this is a spontaneous reaction. Below are the half reactions for sulfate reduction using acetate as a source of electrons, energy, and carbon. - 2CO2+8eCH3COO(0.29 volts) - SO42+8eH2S(0.22 volts) Using the information given, calculate the E for this reaction, balance the full reaction to determine n, the number of electrons transferred when 47 moles of SO42 are reduced. Finally, use the simplified Nernst Equation G=nFE,whereF=96.5kJ(moleV)1 to determine the Gibbs Free energy available to do work! - Report your answer in kJ in standard decimal notation rounded to two decimal places. - Include trailing decimals, even if they are zeros - e.g. 18.00, not 18 - Report only the numeric portion of your answer - e.g. 1.01, not 1.01kj per mole. - Answers should ALWAYS be negative since this is a spontaneous reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


