Question: please answer it correctly A 17.21 gram sample of an organic compound containing C,H and O is analyzed by combustion analysis and 16.83 grams of
please answer it correctly 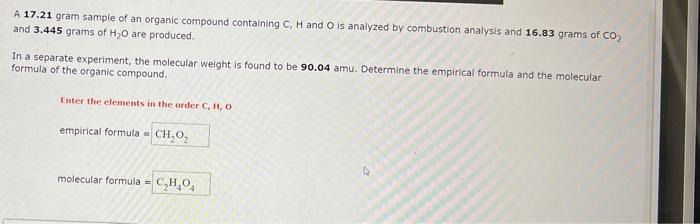

A 17.21 gram sample of an organic compound containing C,H and O is analyzed by combustion analysis and 16.83 grams of CO2 and 3,445 grams of H2O are produced. In a separate experiment, the molecular weight is found to be 90.04 amu. Determine the empirical formula and the molecular formula of the organic compound. Enter the elements in the order C,H,O empirical formula = molecular formula =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


