Question: please answer them correctly A 12.73 gram sample of an organic compound containing C,H and O is analyzed by combustion analysis and 16.21 grams of
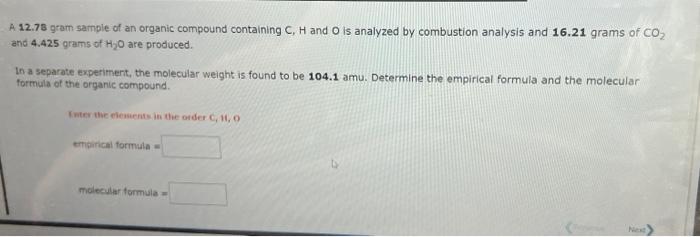
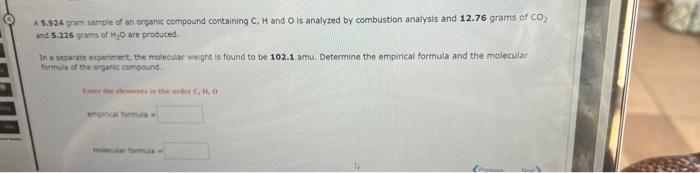


A 12.73 gram sample of an organic compound containing C,H and O is analyzed by combustion analysis and 16.21 grams of CO and 4.425 grams of H2O are produced. In a separate experiment, the molecular weight is found to be 104.1 amu. Determine the empirical formula and the molecular formula of the organic compound. tinitical formitula miclecular formula a 4 5.924 gram sample of an orgaric compound containing C,H and O is analyzed by combustion analysis and 12.76grams of CO2 and 5.226 yrms of Hig are produced in a separate experament, the molecular weight is found to be 102.1 amu. Determine the empirical formula and the molecular formali of the srganic compound. maies ahar formule = Use the References to access important values if needed for this question. According to the following reaction, how many grams of carbon dioxide will be formed upon the complete reaction of 22.69 grams of oxygen gas with excess propane (C3H2H)7 ? propane ( C_3_H_ _ _ Mass = According to the following reaction, how many grams of carbon tetrachloride are required for the complete reaction of 24.8 grams of methane (CH4) ? methune (CH4)(g)+carbon tetrachloride (g) dichloromethane (CH2Cl2)(g) Mass = grams carbon tetrachloride
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


