Question: Please answer it correctly and add details to your solution. Given: A weak acid, HA (whose Ka is 1.00 x 10-6 and Ko is 100)
Please answer it correctly and add details to your solution.
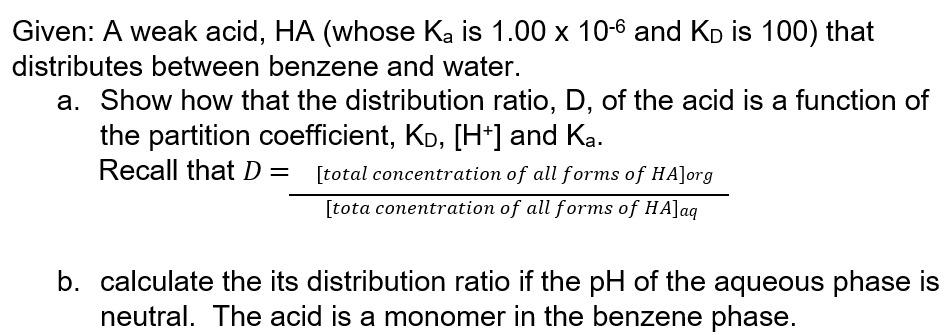
Given: A weak acid, HA (whose Ka is 1.00 x 10-6 and Ko is 100) that distributes between benzene and water. a. Show how that the distribution ratio, D, of the acid is a function of the partition coefficient, Kd, [H+] and Ka. Recall that D= [total concentration of all forms of HA]org [tota conentration of all forms of HA]aq b. calculate the its distribution ratio if the pH of the aqueous phase is neutral. The acid is a monomer in the benzene phase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


