Question: Please answer just part D. Show all process and formula please! 3.36. One cubic meter of argon is taken from 1 bar and 25C to
Please answer just part D. Show all process and formula please!
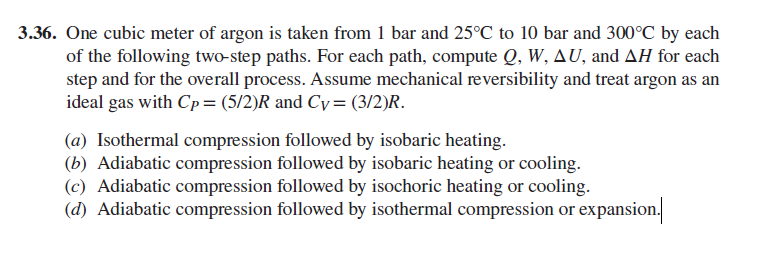
3.36. One cubic meter of argon is taken from 1 bar and 25C to 10 bar and 300C by each of the following two-step paths. For each path, compute Q, W, AU, and AH for each step and for the overall process. Assume mechanical reversibility and treat argon as an ideal gas with Cp= (5/2)R and Cy= (3/2)R. (a) Isothermal compression followed by isobaric heating. (b) Adiabatic compression followed by isobaric heating or cooling. (c) Adiabatic compression followed by isochoric heating or cooling. (d) Adiabatic compression followed by isothermal compression or expansion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


