Question: Please answer part d and e. Given below the temperature and composition data of an octane-toluene (OT) mixture at 1atm. in the table and the
 Please answer part d and e.
Please answer part d and e.
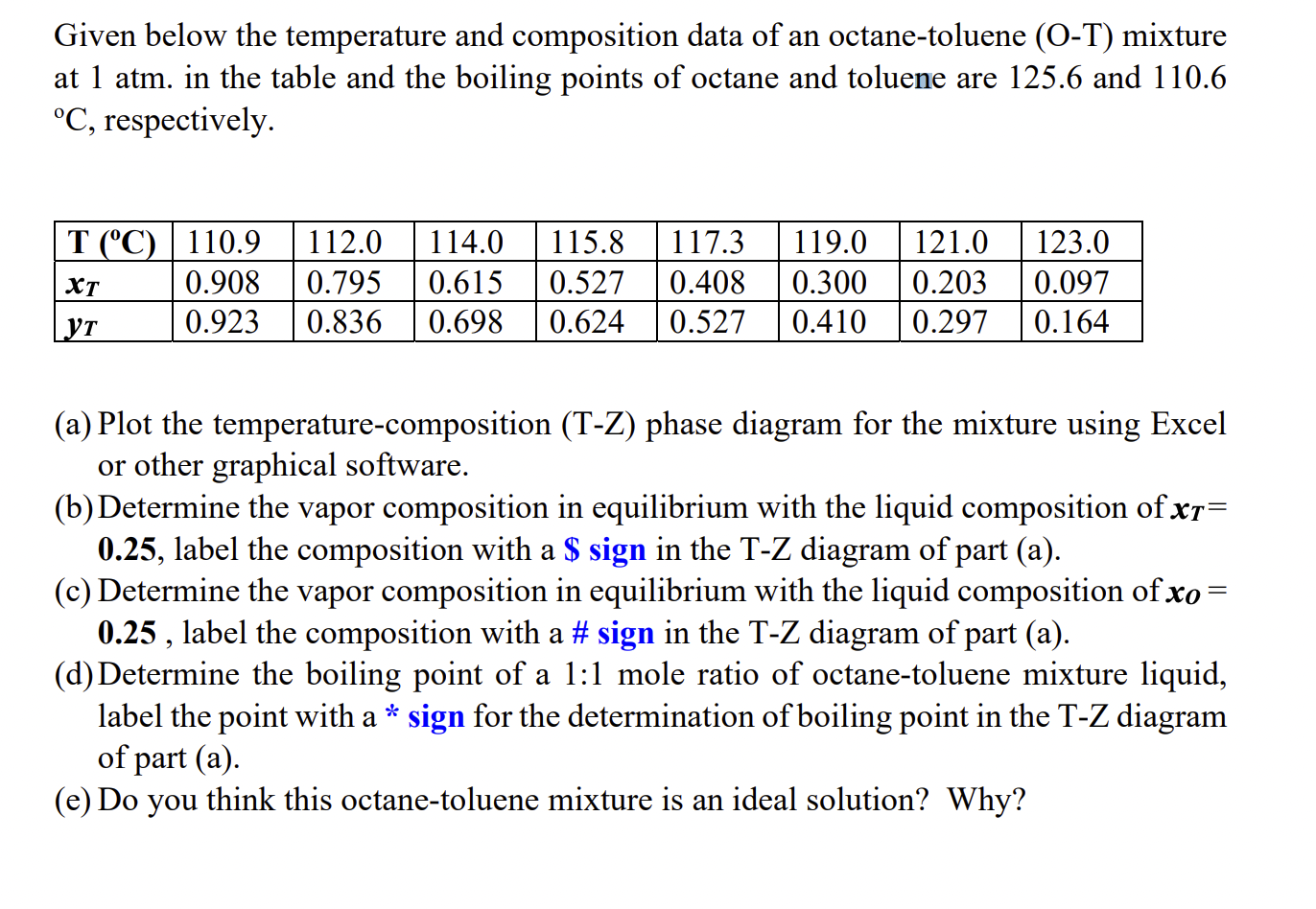
Given below the temperature and composition data of an octane-toluene (OT) mixture at 1atm. in the table and the boiling points of octane and toluene are 125.6 and 110.6 C, respectively. [ (a) Plot the temperature-composition (T-Z) phase diagram for the mixture using Excel or other graphical software. (b) Determine the vapor composition in equilibrium with the liquid composition of xT= 0.25, label the composition with a S sign in the TZ diagram of part (a). (c) Determine the vapor composition in equilibrium with the liquid composition of xo= 0.25, label the composition with a \# sign in the T-Z diagram of part (a). (d) Determine the boiling point of a 1:1 mole ratio of octane-toluene mixture liquid, label the point with asign for the determination of boiling point in the TZ diagram of part (a). (e) Do you think this octane-toluene mixture is an ideal solution? Why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


