Question: Question 3 (Vapor-Liquid equilibrium model, 50 marks) For binary liquid solution containing isopropanol (IPA) - water, a) Construct Tx,y diagram at 101.3kPa; indicate bubble-point curve

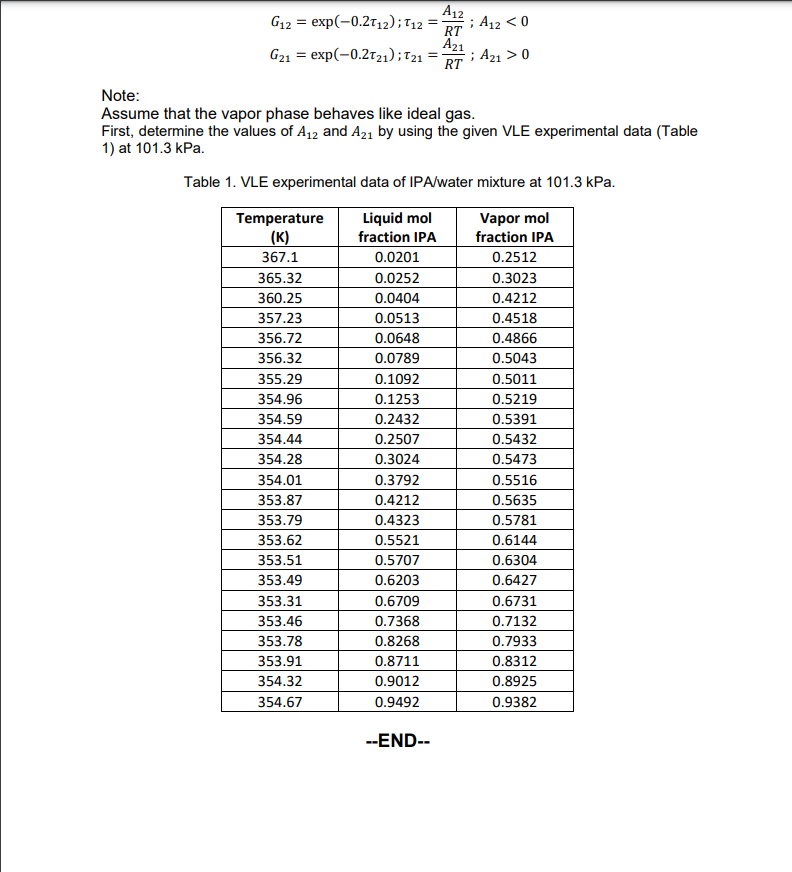
Question 3 (Vapor-Liquid equilibrium model, 50 marks) For binary liquid solution containing isopropanol (IPA) - water, a) Construct Tx,y diagram at 101.3kPa; indicate bubble-point curve and dewpoint curve. In your answer sheet, concisely write down the steps/methods to generate the Tx,y diagram and present the final graph. b) Validate your results in part a) with the given experimental data (see Table 1) and perform error analysis. Referring to the Tx,y diagram in part a): c) Determine the dew point temperature of 80mol% IPA mixture and its liquid composition at the dew point. d) What is the azeotrope boiling point and composition? e) Suppose a liquid solution initially comprises of 30mol IPA is heated up to 360K, determine the resulting liquid and vapor composition. The activity coefficients for IPA (1) and water (2) is expressed by NRTL model as follows: ln1=x22[21(x1+x2G21G21)2+(x2+x1G12)2G1212]ln2=x12[12(x2+x1G12G12)2+(x1+x2G21)2G2121] where: G12=exp(0.212);12=RTA12;A120 Note: Assume that the vapor phase behaves like ideal gas. First, determine the values of A12 and A21 by using the given VLE experimental data (Table 1) at 101.3kPa. Table 1. VLE experimental data of IPA/water mixture at 101.3kPa. G12=exp(0.212);12=RTA12;A120 Note: Assume that the vapor phase behaves like ideal gas. First, determine the values of A12 and A21 by using the given VLE experimental data (Table 1) at 101.3kPa. Table 1. VLE experimental data of IPA/water mixture at 101.3kPa. --END
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


