Question: Please answer parts A, B, C, and D according to this equation below. CuSO 4 (s) Cu 2+ (aq) +SO 4 2- (aq) A) Is
Please answer parts A, B, C, and D according to this equation below.
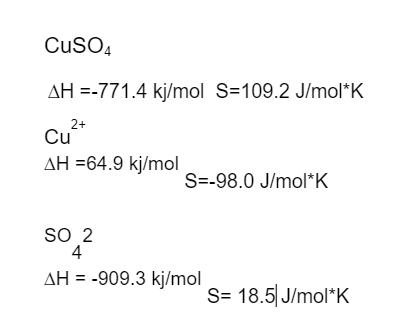
CuSO4 (s) Cu2+ (aq) +SO42-(aq)
A) Is there an increase or decrease in order?
B)Use H and S to calculate G
C) Is it spontaneous? If so, will it be spontaneous for all of the temperatures? If it isn't will the change in spontaneity be above or below 85 C?
D) Why is the entropy of SO42 a positive value but Cu2+ is negative?
CuSO4 AH =-771.4 kj/mol S=109.2 J/mol*K 2+ Cu AH =64.9 kj/mol S=-98.0 J/mol*K SO 2 4 AH = -909.3 kj/mol S= 18.5J/mol*K
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


