Question: Please answer parts A through F below, including a ternary diagram that includes all o f its necessary components and complete the tables below. (
Please answer parts A through below, including a ternary diagram that includes all its necessary components and complete the tables below.
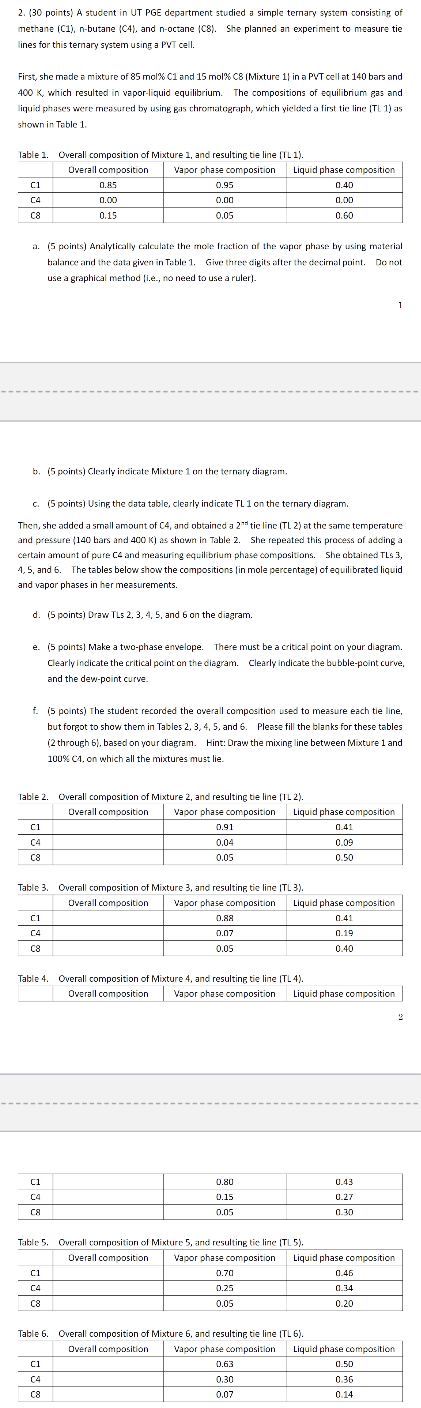
points A student in UT PGE department studied a simple ternary system consisting of methane C nbutane C and noctane C She planned an experiment to measure tie lines for this ternary system using a PVT cell. First, she made a mixture of mol and mol CMixture in a PVT cell at bars and which resulted in vaporliquid equilibrium. The compositions of equilibrium gas and liquid phases were measured by using Eas chromatograph, which yielded a first tie line TL as
shown in Tble
a points Analytically calculate the mole fraction af the vapor phase by using material tralarice and the cata given in Table Give three digits after the decimal point. Do not use a graphical method ie no need to use a ruler
b points Clearly indicate Mixture on the ternary diagram.
c points Usinf, the data table, clearly indicate TL on the ternary diagram. Then, she added a small amount of and obtained a tie line TL at the same temperature and pressure bars and as shown in Table She repeated this process of adding a certain amount of pure and measuring equilibrium phase compositions. She abtained TLS
and The tables below show the compositions in mole percentage of equil brated liquid
and vapor phases in her measurements.
d points Draw TLs and on the diagram.
e points Make a twophase envelope. There must be a critical point on your diagram. Clearly indicate the critical point on the diagram. Clearly indicate the bubblepoint curve, and the dewpoint curve.
f points The student recorded the overall composition used to measure each tie line, but forgot to show them in Tables and Please fill the blanks for these tables through basecl on your diagram. Hint: Oraw the mixing line between Mixture and
on which all the mixtures must lie. Table Overall composition of Mixture and resultine, tie line TL Table Overall composition of Mixture and resulting tie line TL
Table Overall composition of Mixture and resultine tie line TL Table Overall composition of Mixture and resulting tie line TL
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


