Question: Please answer question 2 and 3 asap. c. Tabulate all 2. Using the method of initial rates, determine the reaction order x for hydrogen peroxide.
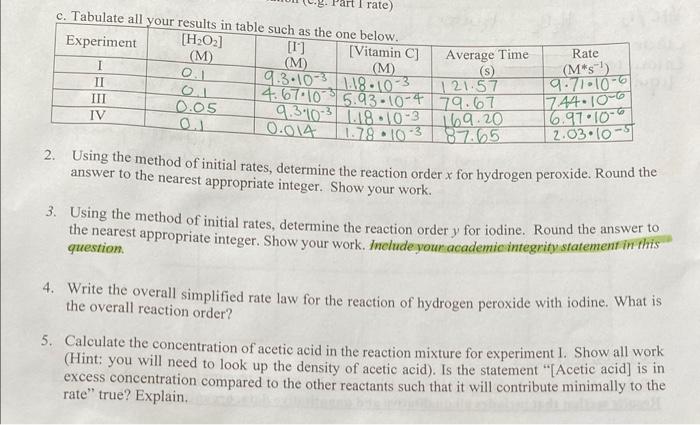
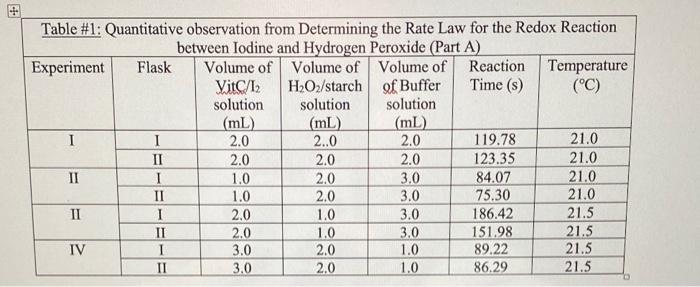

c. Tabulate all 2. Using the method of initial rates, determine the reaction order x for hydrogen peroxide. Round the answer to the nearest appropriate integer. Show your work. 3. Using the method of initial rates, determine the reaction order y for iodine. Round the answer to the nearest appropriate integer. Show your work. Inelude your academicintegrity statement in this question. 4. Write the overall simplified rate law for the reaction of hydrogen peroxide with iodine. What is the overall reaction order? 5. Calculate the concentration of acetic acid in the reaction mixture for experiment 1 . Show all work (Hint: you will need to look up the density of acetic acid). Is the statement "[Acetic acid] is in excess concentration compared to the other reactants such that it will contribute minimally to the rate" true? Explain. Table \#1: Quantitative observation from Determining the Rate Law for the Redox Reaction between Vitamin C and iodine, with a twist. Hydrogen peroxide and iodide ions react relatively slowly according to equation 10 . H2O2(aq)+3I(aq)+2H+(aq)H(aq)+2H2O(1) In the presence of starch, this reaction will gradually turn from colourless to blue-black. But with a Sitamin amount of Vitamin C present, the iodine produced by reaction 10 will convert back to 1- by Only when the Vitamin C is completely consumed will the iodine produced by reaction 10 react with starch, and then the colour change is immediate, starch, and then the colour change is immediate. The iodine used in this experiment is a 2.5% iodine solution which contains 0.098M of 12 and 0.15M of The Vitamin C used in this experiment is from a commercially available tablet containing 500mg of Vitamin C. It was crushed and diluted such that its initial concentration is 6.028M. It is mixed with the The hydrogen peroxide used in this experiment is from a commercially available 3.0% solution and is diluted and mixed with starch indicator for an initial concentration of 0.30M. The rate of the reaction for 10 can be expressed as [H2O2]/t which is the same as [VitC]t because the time it takes for the starch/iodine colour to appear is the same time it takes for all the vitamin C to disappear. The rate law for reaction 10 would be Rate=[H2O2]t=k[H2O2]x[I][H+]2 In this lab, the effect of hydrogen ions will not be studied - the vinegar ( 5% acetic acid by volume) added will act as a buffer and is in excess concentration compared to the other reactants such that it will contribute minimally to the rate. The buffer is also used to maintain total volume of the reaction mixture and the method of initial rates can be applied by changing only volumes of reactants, which effectively changes concentration of reactants via dilution. So, essentially the rate can be calculated according to the equation: Rate=[VitC]/t=k[H2O2]x[I] Once the rate is calculated for different trials, the reaction orders x and y can be determined using the nethod of initial rates. Cemperature Effects: Determination of Activation Energy he Arrhenius equation is k=AeEarT owever, if rearranged into
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


