Question: Please answer question #2 and #3. The topic is kinetics, rate law P73 fx A B D E F G H 1 ] L M
![law P73 fx A B D E F G H 1 ]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d3ba60eb9_16166f8d3b9ae4cb.jpg)
Please answer question #2 and #3. The topic is kinetics, rate law
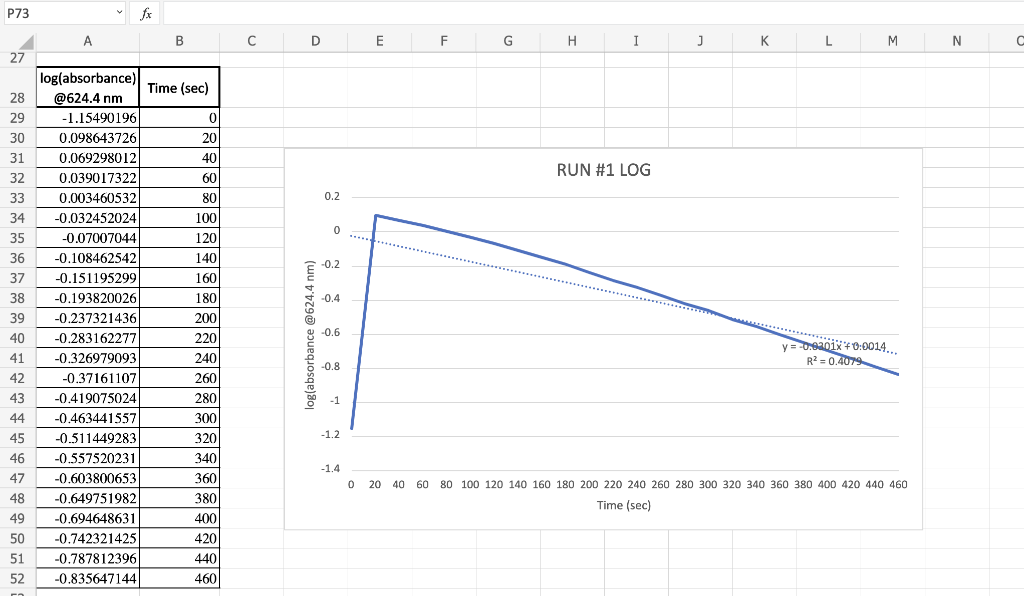
P73 fx A B D E F G H 1 ] L M N C 27 Time (sec) 0 20 40 RUN #1 LOG 60 80 0.2 0 100 1201 140 160 -0.2 -0.4 log(absorbance) @624.4 nm -1.15490196 0.098643726 0.069298012 0.039017322 0.003460532 -0.032452024 -0.07007044 -0.108462542 -0.151195299 -0.193820026 -0.237321436 -0.283162277 -0.326979093 -0.37161107 -0.419075024 -0.463441557 -0.511449283 -0.557520231 -0.603800653 -0.649751982 -0.694648631 -0.742321425 -0.787812396 -0.835647144 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 -0.6 log(absorbance @624.4 nm) y = -0.9901x 40:0014... R?=0.4079 -0.8 -1 180 200 220 240 260 280 300 320 340 360 3801 400 420 4401 460 -1.2 -1.4 0 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 Time (sec) 1/2 27 HOMEWORK QUESTIONS and PROBLEMS 1. You have used a spreadsheet to obtain three full-sized plots of your data from Run 1-plotting Absorbance con they axis) vs time (on the x axis), log of the One of these Absorbance vs time, and reciprocal of the Absorbance vs time. plots should be linear. VS time. a) Which plot is linear? 100 of the absorbance b) Therefore, this is a 1st order reaction in dye; and the slope (in general) is equal to what value that is related to the rate law -0.0301 s'? c) Using the plot which is linear obtain a "best fit" line with the spreadsheet. From the equation of that line record the slope. Show the calculation of Abs, from the y intercept, then the calculation, if one is necessary, of [dye), using E (p. 158). Arm slope = Abs, = (= 1.00cm 8= 1.1x1015 [dye), 2. Prove that the conditions in Run 1 provided a large excess of bleach relative to dye. That is, using your calculated (dye), above, compare it with [bleach), calculated from your answer to Preliminary Question 2 and the amount that the bleach was diluted (see Table A). 3. Using the plot of Absorbance vs time and Abs., read the time for the first half-life of the reaction off of the graph. Then read the time for the second and the third half-lives. 1st half-life 2nd half-life 3rd half-life If this reaction is first order (or pseudo- first order) in dye you should have obtained half-lives that are approximately constant. Does your determination of the half-lives confirm that the reaction is first order in dye, yes or no? P73 fx A B D E F G H 1 ] L M N C 27 Time (sec) 0 20 40 RUN #1 LOG 60 80 0.2 0 100 1201 140 160 -0.2 -0.4 log(absorbance) @624.4 nm -1.15490196 0.098643726 0.069298012 0.039017322 0.003460532 -0.032452024 -0.07007044 -0.108462542 -0.151195299 -0.193820026 -0.237321436 -0.283162277 -0.326979093 -0.37161107 -0.419075024 -0.463441557 -0.511449283 -0.557520231 -0.603800653 -0.649751982 -0.694648631 -0.742321425 -0.787812396 -0.835647144 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 -0.6 log(absorbance @624.4 nm) y = -0.9901x 40:0014... R?=0.4079 -0.8 -1 180 200 220 240 260 280 300 320 340 360 3801 400 420 4401 460 -1.2 -1.4 0 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 Time (sec) 1/2 27 HOMEWORK QUESTIONS and PROBLEMS 1. You have used a spreadsheet to obtain three full-sized plots of your data from Run 1-plotting Absorbance con they axis) vs time (on the x axis), log of the One of these Absorbance vs time, and reciprocal of the Absorbance vs time. plots should be linear. VS time. a) Which plot is linear? 100 of the absorbance b) Therefore, this is a 1st order reaction in dye; and the slope (in general) is equal to what value that is related to the rate law -0.0301 s'? c) Using the plot which is linear obtain a "best fit" line with the spreadsheet. From the equation of that line record the slope. Show the calculation of Abs, from the y intercept, then the calculation, if one is necessary, of [dye), using E (p. 158). Arm slope = Abs, = (= 1.00cm 8= 1.1x1015 [dye), 2. Prove that the conditions in Run 1 provided a large excess of bleach relative to dye. That is, using your calculated (dye), above, compare it with [bleach), calculated from your answer to Preliminary Question 2 and the amount that the bleach was diluted (see Table A). 3. Using the plot of Absorbance vs time and Abs., read the time for the first half-life of the reaction off of the graph. Then read the time for the second and the third half-lives. 1st half-life 2nd half-life 3rd half-life If this reaction is first order (or pseudo- first order) in dye you should have obtained half-lives that are approximately constant. Does your determination of the half-lives confirm that the reaction is first order in dye, yes or no
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


