Question: please answer question 2 in the picture b) On the same drawing label each chemically distinct hydrogen. (3 points) c) How many chemically distinct hydrogens
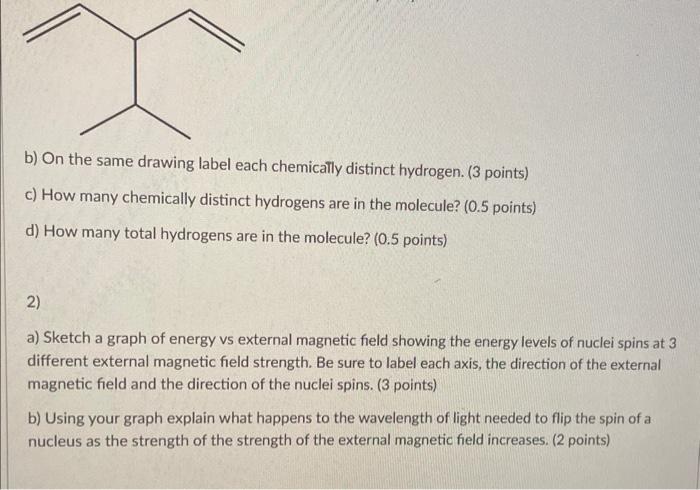
b) On the same drawing label each chemically distinct hydrogen. (3 points) c) How many chemically distinct hydrogens are in the molecule? (0.5 points) d) How many total hydrogens are in the molecule? ( 0.5 points) 2) a) Sketch a graph of energy vs external magnetic field showing the energy levels of nuclei spins at 3 different external magnetic field strength. Be sure to label each axis, the direction of the external magnetic field and the direction of the nuclei spins. (3 points) b) Using your graph explain what happens to the wavelength of light needed to flip the spin of a nucleus as the strength of the strength of the external magnetic field increases. (2 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


