Question: PLEASE ANSWER QUESTION WITH CLEAR ANSWERS, NOT ONLY A GUIDE ON HOW TO SOLVE THEM. 1. At 200C, benzene et toluene form an ideal solution,
PLEASE ANSWER QUESTION WITH CLEAR ANSWERS, NOT ONLY A GUIDE ON HOW TO SOLVE THEM.
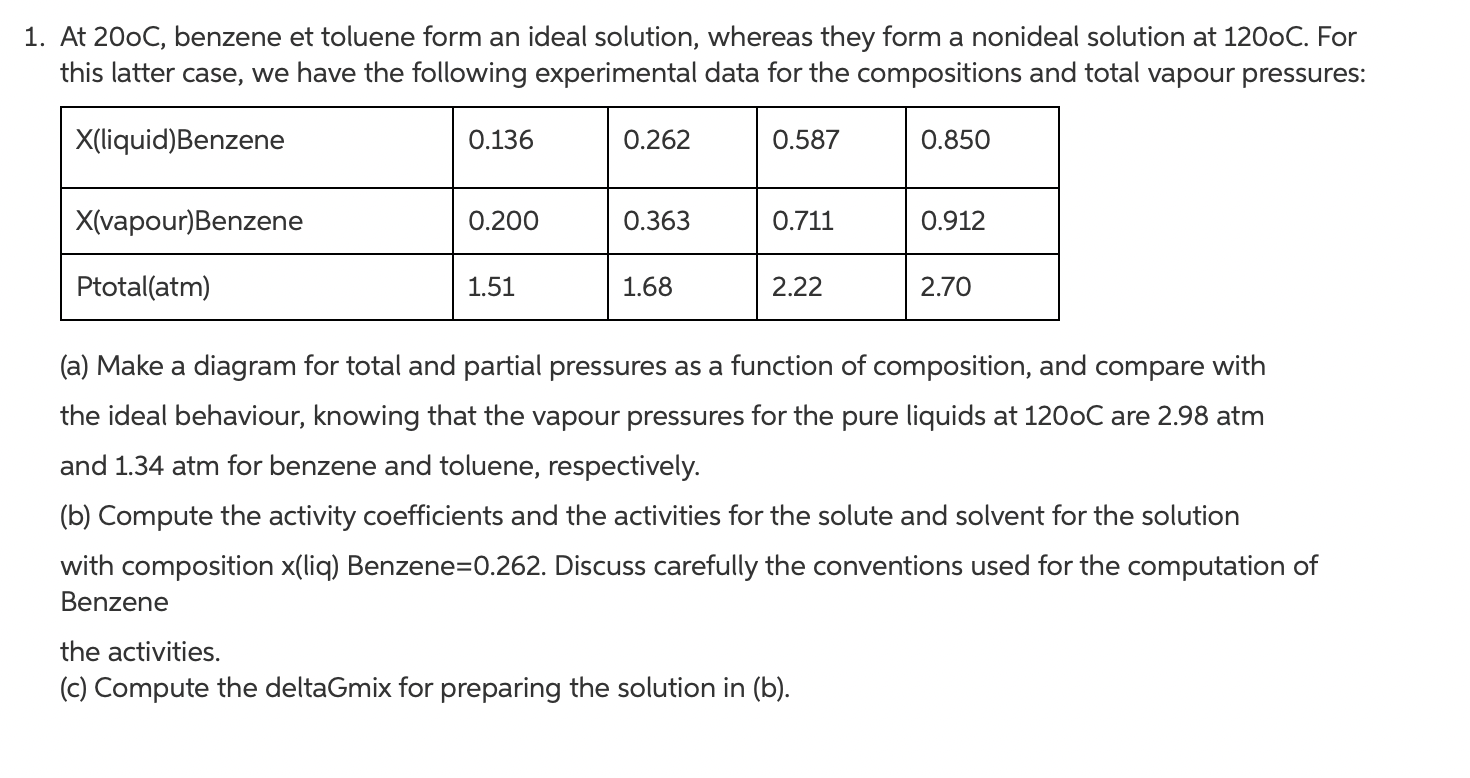
1. At 200C, benzene et toluene form an ideal solution, whereas they form a nonideal solution at 1200C. For this latter case, we have the following experimental data for the compositions and total vapour pressures: X(liquid)Benzene 0.136 0.262 0.587 0.850 X(vapour)Benzene 0.200 0.363 0.711 0.912 Ptotal(atm) 1.51 1.68 2.22 2.70 (a) Make a diagram for total and partial pressures as a function of composition, and compare with the ideal behaviour, knowing that the vapour pressures for the pure liquids at 1200C are 2.98 atm and 1.34 atm for benzene and toluene, respectively. (b) Compute the activity coefficients and the activities for the solute and solvent for the solution with composition x(liq) Benzene=0.262. Discuss carefully the conventions used for the computation of Benzene the activities. (c) Compute the deltaGmix for preparing the solution in (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


