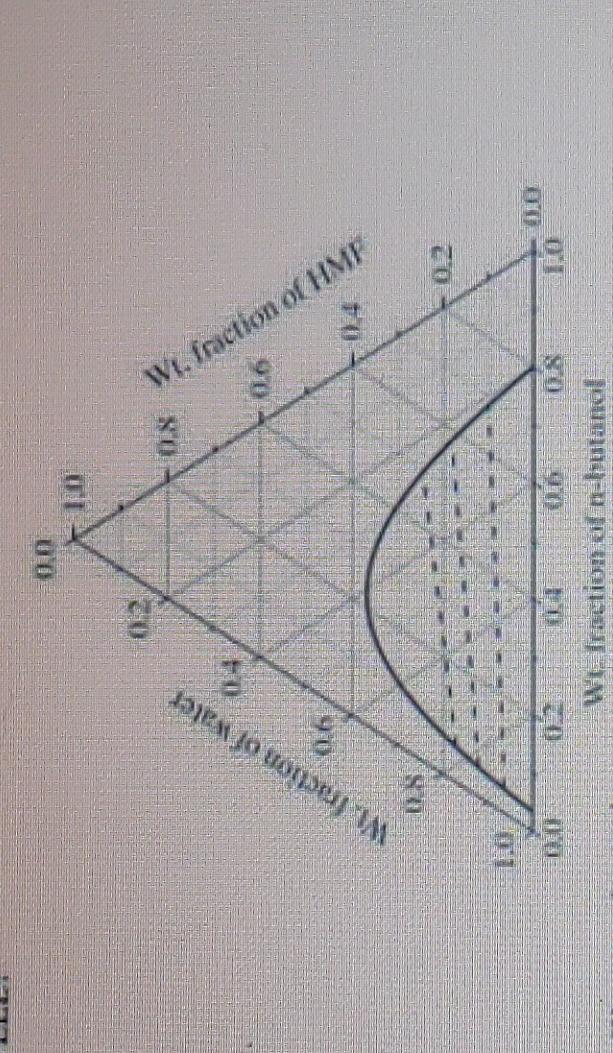
Question: please answer question with full explanation For mixture containing 0.12kg of water, 0.027kg of HMF and 0.153kg of n-butanol, the compositions of the phases present

please answer question with full explanation
For mixture containing 0.12kg of water, 0.027kg of HMF and 0.153kg of n-butanol, the compositions of the phases present in equilibrium are: (a) Water =0.22; n-butanol =0.68;HMF=0.10 and Water =0.90;-butanol =0.04; HMF=0.06 (b) Water =0.10;n-butanol =0.68;HMF=0.22 and Water =0.06;n-butanol =0.04 HMF=0.90 (c). Water =0.22;n-butanol =0.10;HMF=0.68 and Water =0.90;n-butanol =0.10; HMF=0.68 (d) Water =0.68; n-butanol =0.22;HMF=0.10 and Water =0.04; n-butanol =0.90; HMF=0.06
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


