Question: Please answer quickly(within 1 hour) and correctly for upvote and share to friends. A volume of 1m3 of carbon dioxide (CO2) initially at 150C and
Please answer quickly(within 1 hour) and correctly for upvote and share to friends.
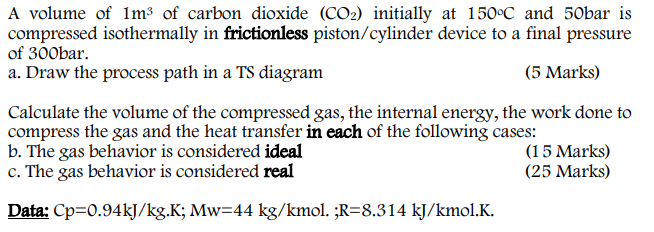
A volume of 1m3 of carbon dioxide (CO2) initially at 150C and 50bar is compressed isothermally in frictionless piston/cylinder device to a final pressure of 300bar. a. Draw the process path in a TS diagram (5 Marks) Calculate the volume of the compressed gas, the internal energy, the work done to compress the gas and the heat transfer in each of the following cases: b. The gas behavior is considered ideal (15 Marks) c. The gas behavior is considered real (25 Marks) Data: Cp=0.94kJ/kg.K; Mw=44 kg/kmol. ;R=8.314 kJ/kmol.K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


