Question: please answer read it all the info is there Pharma is a family owned and operated pharmaceutical company with a corporate office and a production
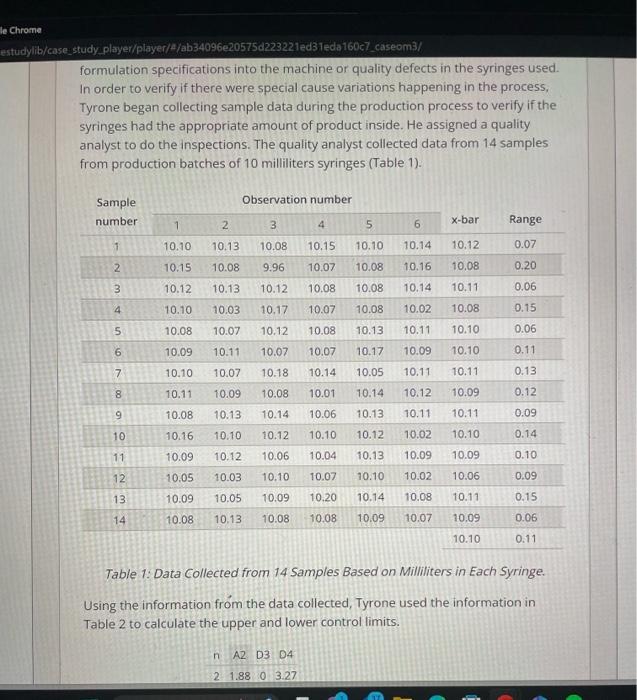
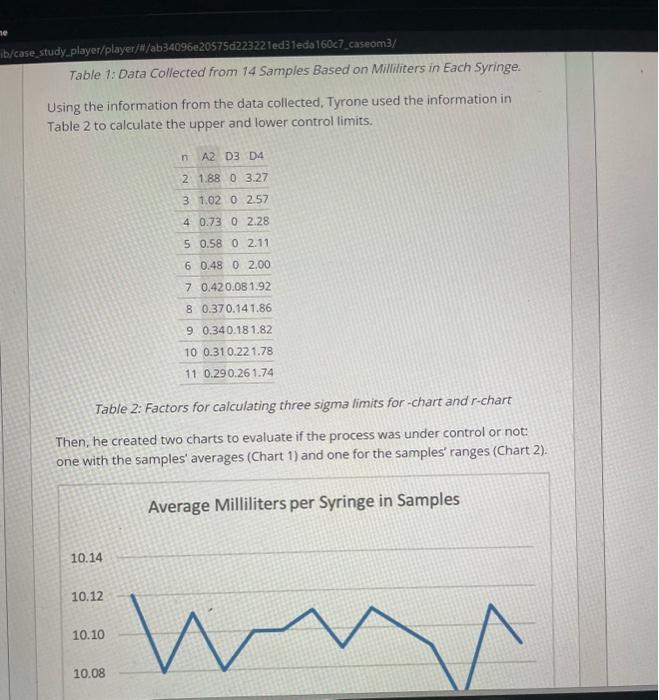
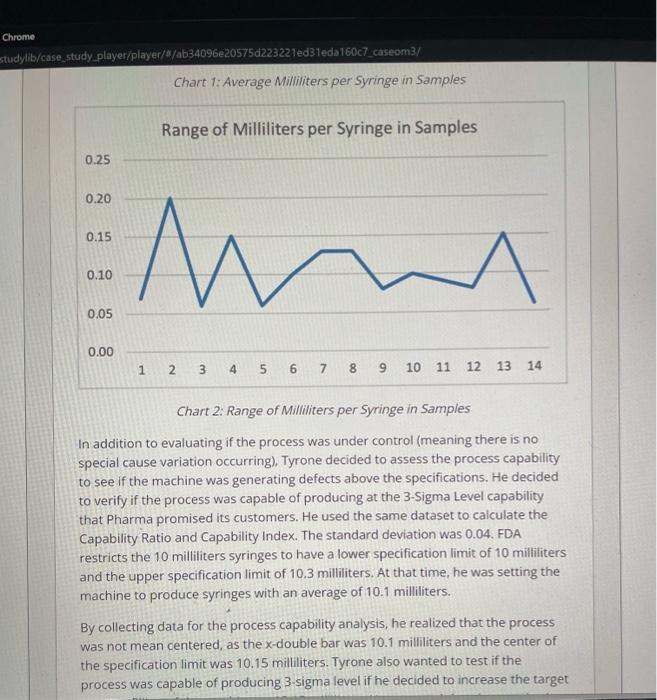

Pharma is a family owned and operated pharmaceutical company with a corporate office and a production facility in South Carolina. It is a leader in manufacturing generic respiratory medication and contract manufacturing. As a pharmaceutical company, Pharma's operations are heavily restricted by the strict guidelines of the Food and Drug Administration (FDA), a branch of the US federal government. Pharma receives orders from drug wholesale companies, which distribute the products to hospitals, retail pharmacies, mail order pharmacies, home care companies, and long-term care facilities. Pharma purchases medications and packaging in large quantities and mixes and packages the medications according to the needs of the customers. The medications can be packaged in syringes, bags, or ampoules, with dozens of different variations of sizes and concentrations. Tyrone Smith is the Operations Manager for the production department responsible for the automated syringe filling machines. The machines are in a sterile room and need two production operators. One operator loads the equipment with syringes and makes sure that the process is running as planned, and the other one unloads the boxes with syringes. Pharma's production processes have to comply with strict FDA regulations. First, the room with the equipment has to be sterilized. The operators then have to go through a detailed cleaning process and wear special sterile clothes. When they begin production, the first samples have to be tested in a specialized microbiology lab. After the lab confirms that the batch is okay for production, an environmental technician has to certify that the air in the room is sterile and has no particulates. Last, after the batch is finalized, another team has to go inside the room and perform a deep cleaning before they can begin the next batch. Problem Definition One day, Tyrone was called for an emergency meeting with representatives from customer service, quality, supply chain operations, and upper management. A batch produced by Tyrone's department a few weeks ago was rejected by a large One day, Tyrone was called for an emergency meeting with representatives from customer service, quality, supply chain operations, and upper management. A batch produced by Tyrone's department a few weeks ago was rejected by a large customer. The customer told Pharma that the filling quantity was lower than the specifications for a 10-milliliter syringe. This was a serious problem for Pharma, because if the syringe has less medication than specified the patient could receive a lower dose than the one needed. Pharma could recall the batch of syringes and decide either to scrap them or to perform an item-by-item evaluation to see if some of the syringes satisfied the specifications and could be sold again. If they do not recall the syringes, Pharma risks receiving a fine from the FDA that can easily top several million dollars. The recall cost was estimated to be about $150,000. This cost refers to locating the defective syringes and bringing those items back to the manufacturing facility. In addition, they would have to perform an additional quality check of the existing inventory and expedite shipments of new products to all customers. Expedited shipments have a higher cost than normal shipments, as they have to be done overnight in small quantities instead of full truck loads with other products. When the product from the rejected batch arrives at the facility, they would have to decide if they were going to do an item-by-item quality evaluation, initiate an internal rework process, or just scrap the whole batch. The item-by-item evaluation would cost $30,000, but it could generate additional revenue between $40,000 and $90,000, depending in how many products pass the specification limits and could be resold to customers. Assessing Control and Process Capability Tyrone left the meeting very frustrated and determined to find out the reasons behind the rejected batch. He decided to investigate the problem from two different angles. First, he thought that it might be a specific problem that occurred during the days that batch was produced, such as a human error in putting the formulation specifications into the machine or quality defects in the syringes used. formulation specifications into the machine or quality defects in the syringes used. In order to verify if there were special cause variations happening in the process, Tyrone began collecting sample data during the production process to verify if the syringes had the appropriate amount of product inside. He assigned a quality analyst to do the inspections. The quality analyst collected data from 14 samples from production batches of 10 milliliters syringes (Table 1). Table 1: Data Collected from 14 Samples Based on Milliliters in Each Syringe. Using the information from the data collected, Tyrone used the information in Table 2 to calculate the upper and lower control limits. Table 1: Data Collected from 14 Samples Based on Milliliters in Each Syringe. Using the information from the data collected, Tyrone used the information in Table 2 to calculate the upper and lower control limits. Table 2: Factors for calculating three sigma limits for-chart and r-chart Then, he created two charts to evaluate if the process was under control or not: one with the samples' averages (Chart 1) and one for the samples' ranges (Chart 2). Chart 2: Range of Milliliters per Syringe in Samples In addition to evaluating if the process was under control (meaning there is no special cause variation occurring), Tyrone decided to assess the process capability to see if the machine was generating defects above the specifications. He decided to verify if the process was capable of producing at the 3-Sigma Level capability that Pharma promised its customers. He used the same dataset to caiculate the Capability Ratio and Capability Index. The standard deviation was 0.04 . FDA restricts the 10 milliliters syringes to have a lower specification limit of 10 milliliters and the upper specification limit of 10.3 milliiters. At that time, he was setting the machine to produce syringes with an average of 10.1 milliliters. By collecting data for the process capability analysis, he realized that the process was not mean centered, as the x-double bar was 10.1 milliliters and the center of the specification limit was 10.15 milliliters. Tyrone also wanted to test if the process was capable of producing 3-sigma level if he decided to increase the target In addition to evaluating if the process was under control (meaning there is no special cause variation occurring). Tyrone decided to assess the process capability to see if the machine was generating defects above the specifications. He decided to verify if the process was capable of producing at the 3-Sigma Level capability that Pharma promised its customers. He used the same dataset to calculate the Capability Ratio and Capability index. The standard deviation was 0.04. FDA restricts the 10 milliliters syringes to have a lower specification limit of 10 milliliters and the upper specification limit of 10.3 milliliters. At that time, he was setting the machine to produce syringes with an average of 10.1 milliliters. By collecting data for the process capability analysis, he realized that the process was not mean centered, as the x-double bar was 10.1 milliliters and the center of the specification limit was 10.15 milliliters. Tyrone also wanted to test if the process was capable of producing 3-sigma level if he decided to increase the target average filling of the machine to 10.15 milliliters. He expected that the standard deviation would not change. In addition, he decided to retrain all his employees in how to set the machine in order to prevent future quality problems from happening. Pharma Manufacturing Quality Management Investigation After Customer Rejection To complete your assignment, read the case study on the Brief tab and provide your responses in the Questions tab. You can switch between tabs while working to review the case brief. Was the process under control and capable of producing at 3-sigma level? Calculate the upper and lower control limits, capability ratio, and index for the current situation and for the average of 10.15 milliliters to justify your answer. You can use Charts 1 and 2 to draw the UCLS and LCLS. Pharma is a family owned and operated pharmaceutical company with a corporate office and a production facility in South Carolina. It is a leader in manufacturing generic respiratory medication and contract manufacturing. As a pharmaceutical company, Pharma's operations are heavily restricted by the strict guidelines of the Food and Drug Administration (FDA), a branch of the US federal government. Pharma receives orders from drug wholesale companies, which distribute the products to hospitals, retail pharmacies, mail order pharmacies, home care companies, and long-term care facilities. Pharma purchases medications and packaging in large quantities and mixes and packages the medications according to the needs of the customers. The medications can be packaged in syringes, bags, or ampoules, with dozens of different variations of sizes and concentrations. Tyrone Smith is the Operations Manager for the production department responsible for the automated syringe filling machines. The machines are in a sterile room and need two production operators. One operator loads the equipment with syringes and makes sure that the process is running as planned, and the other one unloads the boxes with syringes. Pharma's production processes have to comply with strict FDA regulations. First, the room with the equipment has to be sterilized. The operators then have to go through a detailed cleaning process and wear special sterile clothes. When they begin production, the first samples have to be tested in a specialized microbiology lab. After the lab confirms that the batch is okay for production, an environmental technician has to certify that the air in the room is sterile and has no particulates. Last, after the batch is finalized, another team has to go inside the room and perform a deep cleaning before they can begin the next batch. Problem Definition One day, Tyrone was called for an emergency meeting with representatives from customer service, quality, supply chain operations, and upper management. A batch produced by Tyrone's department a few weeks ago was rejected by a large One day, Tyrone was called for an emergency meeting with representatives from customer service, quality, supply chain operations, and upper management. A batch produced by Tyrone's department a few weeks ago was rejected by a large customer. The customer told Pharma that the filling quantity was lower than the specifications for a 10-milliliter syringe. This was a serious problem for Pharma, because if the syringe has less medication than specified the patient could receive a lower dose than the one needed. Pharma could recall the batch of syringes and decide either to scrap them or to perform an item-by-item evaluation to see if some of the syringes satisfied the specifications and could be sold again. If they do not recall the syringes, Pharma risks receiving a fine from the FDA that can easily top several million dollars. The recall cost was estimated to be about $150,000. This cost refers to locating the defective syringes and bringing those items back to the manufacturing facility. In addition, they would have to perform an additional quality check of the existing inventory and expedite shipments of new products to all customers. Expedited shipments have a higher cost than normal shipments, as they have to be done overnight in small quantities instead of full truck loads with other products. When the product from the rejected batch arrives at the facility, they would have to decide if they were going to do an item-by-item quality evaluation, initiate an internal rework process, or just scrap the whole batch. The item-by-item evaluation would cost $30,000, but it could generate additional revenue between $40,000 and $90,000, depending in how many products pass the specification limits and could be resold to customers. Assessing Control and Process Capability Tyrone left the meeting very frustrated and determined to find out the reasons behind the rejected batch. He decided to investigate the problem from two different angles. First, he thought that it might be a specific problem that occurred during the days that batch was produced, such as a human error in putting the formulation specifications into the machine or quality defects in the syringes used. formulation specifications into the machine or quality defects in the syringes used. In order to verify if there were special cause variations happening in the process, Tyrone began collecting sample data during the production process to verify if the syringes had the appropriate amount of product inside. He assigned a quality analyst to do the inspections. The quality analyst collected data from 14 samples from production batches of 10 milliliters syringes (Table 1). Table 1: Data Collected from 14 Samples Based on Milliliters in Each Syringe. Using the information from the data collected, Tyrone used the information in Table 2 to calculate the upper and lower control limits. Table 1: Data Collected from 14 Samples Based on Milliliters in Each Syringe. Using the information from the data collected, Tyrone used the information in Table 2 to calculate the upper and lower control limits. Table 2: Factors for calculating three sigma limits for-chart and r-chart Then, he created two charts to evaluate if the process was under control or not: one with the samples' averages (Chart 1) and one for the samples' ranges (Chart 2). Chart 2: Range of Milliliters per Syringe in Samples In addition to evaluating if the process was under control (meaning there is no special cause variation occurring), Tyrone decided to assess the process capability to see if the machine was generating defects above the specifications. He decided to verify if the process was capable of producing at the 3-Sigma Level capability that Pharma promised its customers. He used the same dataset to caiculate the Capability Ratio and Capability Index. The standard deviation was 0.04 . FDA restricts the 10 milliliters syringes to have a lower specification limit of 10 milliliters and the upper specification limit of 10.3 milliiters. At that time, he was setting the machine to produce syringes with an average of 10.1 milliliters. By collecting data for the process capability analysis, he realized that the process was not mean centered, as the x-double bar was 10.1 milliliters and the center of the specification limit was 10.15 milliliters. Tyrone also wanted to test if the process was capable of producing 3-sigma level if he decided to increase the target In addition to evaluating if the process was under control (meaning there is no special cause variation occurring). Tyrone decided to assess the process capability to see if the machine was generating defects above the specifications. He decided to verify if the process was capable of producing at the 3-Sigma Level capability that Pharma promised its customers. He used the same dataset to calculate the Capability Ratio and Capability index. The standard deviation was 0.04. FDA restricts the 10 milliliters syringes to have a lower specification limit of 10 milliliters and the upper specification limit of 10.3 milliliters. At that time, he was setting the machine to produce syringes with an average of 10.1 milliliters. By collecting data for the process capability analysis, he realized that the process was not mean centered, as the x-double bar was 10.1 milliliters and the center of the specification limit was 10.15 milliliters. Tyrone also wanted to test if the process was capable of producing 3-sigma level if he decided to increase the target average filling of the machine to 10.15 milliliters. He expected that the standard deviation would not change. In addition, he decided to retrain all his employees in how to set the machine in order to prevent future quality problems from happening. Pharma Manufacturing Quality Management Investigation After Customer Rejection To complete your assignment, read the case study on the Brief tab and provide your responses in the Questions tab. You can switch between tabs while working to review the case brief. Was the process under control and capable of producing at 3-sigma level? Calculate the upper and lower control limits, capability ratio, and index for the current situation and for the average of 10.15 milliliters to justify your answer. You can use Charts 1 and 2 to draw the UCLS and LCLS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


