Question: Please answer soon thanks luv u Nitrogen dioxide (NO2) gas and liquid water (H2O) react to form aqueous nitric acid (HNO ) and nitrogen monoxide
Please answer soon thanks luv u 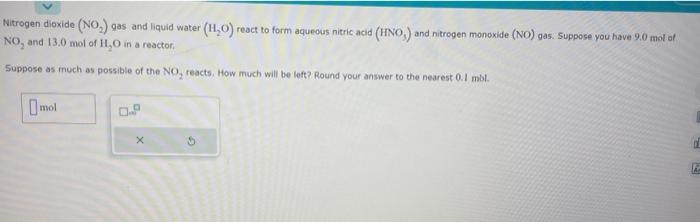

Nitrogen dioxide (NO2) gas and liquid water (H2O) react to form aqueous nitric acid (HNO ) and nitrogen monoxide (NO) gas. Suppose you have 9.0 mol of NO2 and 13.0mol of H2O in a reactor: Suppose as much as possible of the NO2 reacts. How much will be left? Round your answer to the nearest 0.1 mbl
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


