Question: Please answer the following 3) A copper block with an initial temperature of 14C is dropped Into 512g of water initially at 74 C, and
Please answer the following
3) A copper block with an initial temperature of 14C is dropped Into 512g of water initially at 74 C, and they reach an equilibrium temperature of 60 C. Determine the mass of the copper block.
4) you drop 381 grams of iron at a initial temperature of 93 C into 583 grams of 4C Water and wait for them to achieve thermal equilibrium. What is the final temperture of the water?
5) how many joules of heat does it take to melt 121 grams of ice that had a initial temperture of -12 C ? (H f = 333 j/g)

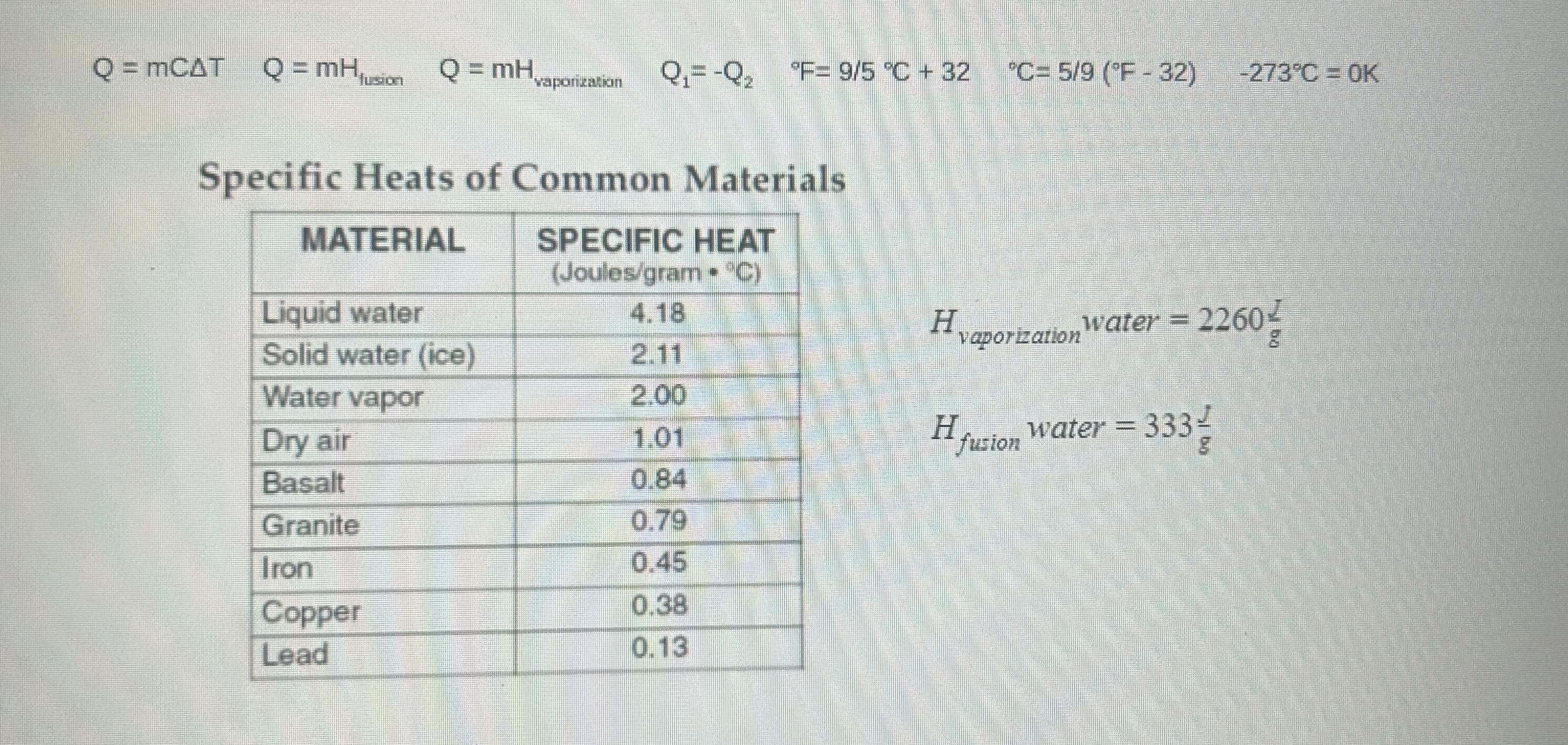
copper block. A copper block with an initial temperature of 14C is dropped into 512g of water initially at 740C, and they reach an equilibrium temperature of 60 C. Determine the mass of the 4 You drop 381 grams of iron at an initial temperature of 93 C into 583 grams of 4 C water and wait for them to achieve thermal equilibrium. What is the final temperature of the water? S How many joules of heat does it take to melt 121 grams of ice that has an initial temperature of -12 C? (H,-333 J/g)Q = mCAT Q=mh Q.= -Q2 "F= 9/5 "C + 32 C= 5/9 ("F - 32) -273.C = OK Specific Heats of Common Materials MATERIAL SPECIFIC HEAT (Joules/gram - "C) Liquid water 4.18 vaporization Water = 22604 Solid water (ice) 2.11 Water vapor 2.00 Dry air 1.01 Hfusion Water = 333- Basalt 0.84 Granite 0.79 Iron 0.45 Copper 0.38 Lead 0. 13
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


