Question: please answer The Ka values for four acids are: H,CO3 4.5 x 10- HCOOH 1.8 x 104 H, C, H. OH(COO)3 7.1 x 104 HCN
please answer
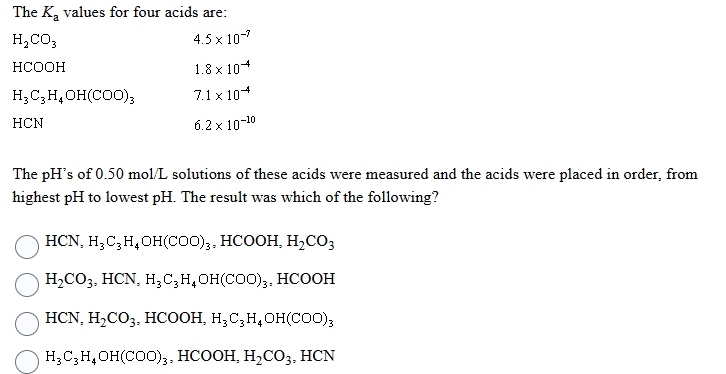
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


