Question: Please Answer the Problem Carefully. A saturated solution of Na2CO3 at 25C is sent to a crystallizer where it is cooled to 15C. The wet
 Please Answer the Problem Carefully.
Please Answer the Problem Carefully.
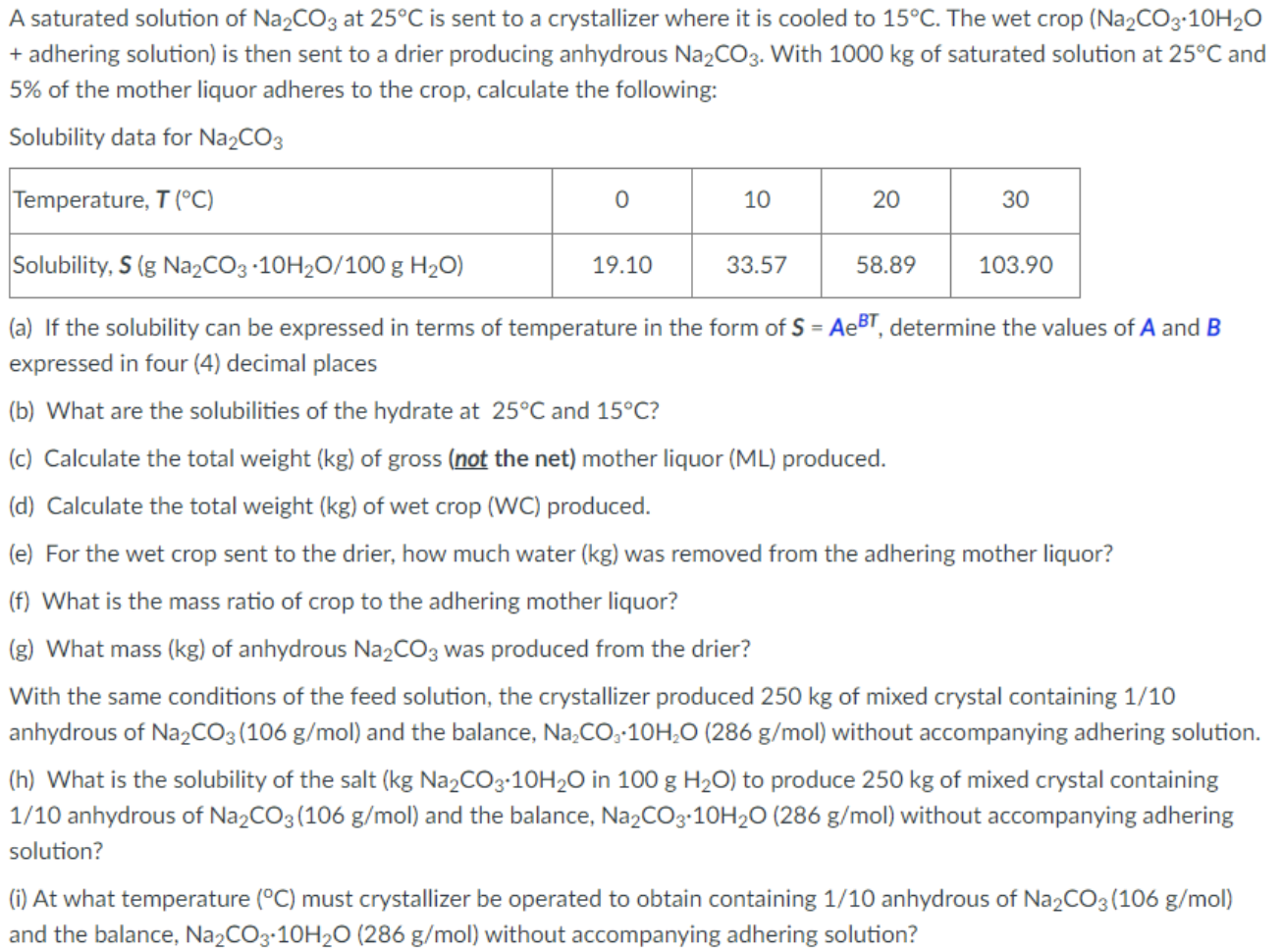
A saturated solution of Na2CO3 at 25C is sent to a crystallizer where it is cooled to 15C. The wet crop (Na2CO3.10H20 + adhering solution) is then sent to a drier producing anhydrous Na2CO3. With 1000 kg of saturated solution at 25C and 5% of the mother liquor adheres to the crop, calculate the following: Solubility data for Na2CO3 Temperature, T (C) 0 10 20 30 Solubility, S (g Na2CO3 -10H20/100 g H20) 19.10 33.57 58.89 103.90 (a) If the solubility can be expressed in terms of temperature in the form of S = AeBT, determine the values of A and B expressed in four (4) decimal places (b) What are the solubilities of the hydrate at 25C and 15C? (c) Calculate the total weight (kg) of gross (not the net) mother liquor (ML) produced. (d) Calculate the total weight (kg) of wet crop (WC) produced. (e) For the wet crop sent to the drier, how much water (kg) was removed from the adhering mother liquor? (f) What is the mass ratio of crop to the adhering mother liquor? (g) What mass (kg) of anhydrous Na2CO3 was produced from the drier? With the same conditions of the feed solution, the crystallizer produced 250 kg of mixed crystal containing 1/10 anhydrous of Na2CO3 (106 g/mol) and the balance, Na.Coz-104,0 (286 g/mol) without accompanying adhering solution. (h) What is the solubility of the salt (kg Na2CO3.10H20 in 100 g H20) to produce 250 kg of mixed crystal containing 1/10 anhydrous of Na2CO3 (106 g/mol) and the balance, Na2CO3.10H20 (286 g/mol) without accompanying adhering solution? (i) At what temperature (C) must crystallizer be operated to obtain containing 1/10 anhydrous of Na2CO3(106 g/mol) and the balance, Na2CO3-10H20 (286 g/mol) without accompanying adhering solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


