Question: Please answer the question completely. Write legibly as well. 3. Enzyme reactions occur by a binding interaction between the enzyme and the reactant. The kinetics
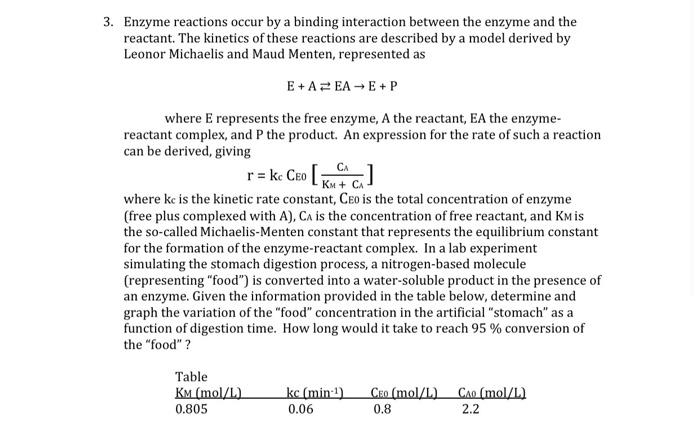
3. Enzyme reactions occur by a binding interaction between the enzyme and the reactant. The kinetics of these reactions are described by a model derived by Leonor Michaelis and Maud Menten, represented as E+AEAE+P CA where E represents the free enzyme, A the reactant, EA the enzyme- reactant complex, and P the product. An expression for the rate of such a reaction can be derived, giving r= ke CEO [kuca] KM + CA where kc is the kinetic rate constant, Ceo is the total concentration of enzyme (free plus complexed with A), C is the concentration of free reactant, and Kmis the so-called Michaelis-Menten constant that represents the equilibrium constant for the formation of the enzyme-reactant complex. In a lab experiment simulating the stomach digestion process, a nitrogen-based molecule (representing "food") is converted into a water-soluble product in the presence of an enzyme. Given the information provided in the table below, determine and graph the variation of the "food" concentration in the artificial "stomach" as a function of digestion time. How long would it take to reach 95 % conversion of the "food" ? Table KM (mol/L). 0.805 kc (min-1) 0.06 Ceo (mol/L) 0.8 Cao (mol/L) 2.2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


