Question: please answer the question fully and completely 4. Reversible reactions. The forward and reverse rate constants (in appropriate units of moles, Liters, and min) for
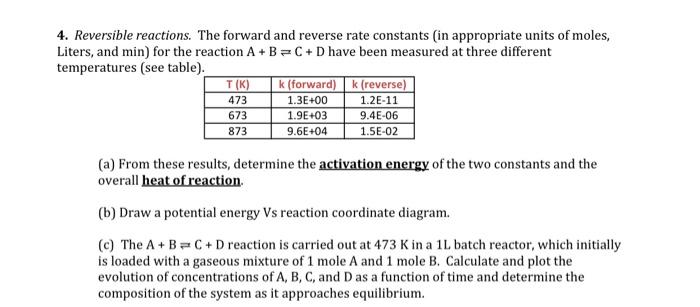
4. Reversible reactions. The forward and reverse rate constants (in appropriate units of moles, Liters, and min) for the reaction A+B=C + D have been measured at three different temperatures (see table). k (forward)k (reverse) 473 1.3E+00 1.2E-11 T(K) 673 873 1.9E+03 9.6E+04 9.4E-06 1.5E-02 (a) From these results, determine the activation energy of the two constants and the overall heat of reaction. (b) Draw a potential energy Vs reaction coordinate diagram. (c) The A+B=C + D reaction is carried out at 473 K in a 1L batch reactor, which initially is loaded with a gaseous mixture of 1 mole A and 1 mole B. Calculate and plot the evolution of concentrations of A, B, C, and Das a function of time and determine the composition of the system as it approaches equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


