Question: Please answer the question fully and since this problem include excel please add pictures of your excel file. Write legibly as well 111 The following
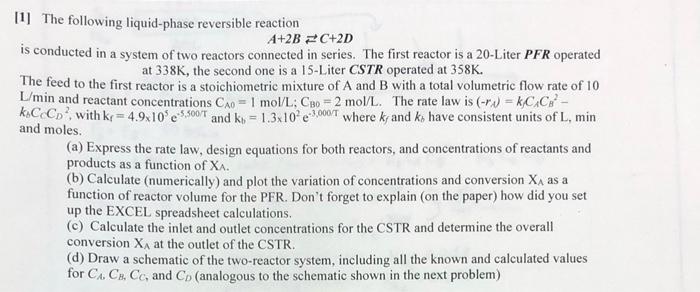
111 The following liquid-phase reversible reaction A+2B = C+2D is conducted in a system of two reactors connected in series. The first reactor is a 20-Liter PFR operated at 338K, the second one is a 15-Liter CSTR operated at 358K. The feed to the first reactor is a stoichiometric mixture of A and B with a total volumetric flow rate of 10 L'min and reactant concentrations Cxo = 1 mol/L; Cao = 2 mol/L. The rate law is (-rs) = k/C/C: - k.C.Co? with kr = 4.9x10.5.500/1 and ko = 1.3x105,000/where kr and ky have consistent units of L, min and moles. (a) Express the rate law, design equations for both reactors, and concentrations of reactants and products as a function of XA. (b) Calculate (numerically) and plot the variation of concentrations and conversion XA as a function of reactor volume for the PFR. Don't forget to explain (on the paper) how did you set up the EXCEL spreadsheet calculations. (c) Calculate the inlet and outlet concentrations for the CSTR and determine the overall conversion XA at the outlet of the CSTR. (d) Draw a schematic of the two-reactor system, including all the known and calculated values for CA CB Cc, and C) (analogous to the schematic shown in the next problem) 111 The following liquid-phase reversible reaction A+2B = C+2D is conducted in a system of two reactors connected in series. The first reactor is a 20-Liter PFR operated at 338K, the second one is a 15-Liter CSTR operated at 358K. The feed to the first reactor is a stoichiometric mixture of A and B with a total volumetric flow rate of 10 L'min and reactant concentrations Cxo = 1 mol/L; Cao = 2 mol/L. The rate law is (-rs) = k/C/C: - k.C.Co? with kr = 4.9x10.5.500/1 and ko = 1.3x105,000/where kr and ky have consistent units of L, min and moles. (a) Express the rate law, design equations for both reactors, and concentrations of reactants and products as a function of XA. (b) Calculate (numerically) and plot the variation of concentrations and conversion XA as a function of reactor volume for the PFR. Don't forget to explain (on the paper) how did you set up the EXCEL spreadsheet calculations. (c) Calculate the inlet and outlet concentrations for the CSTR and determine the overall conversion XA at the outlet of the CSTR. (d) Draw a schematic of the two-reactor system, including all the known and calculated values for CA CB Cc, and C) (analogous to the schematic shown in the next problem)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


