Question: Please answer the question fully and write legibly 2. Membrane reactor. The reversible first order reaction ASB+ 2 C is carried out in the gas
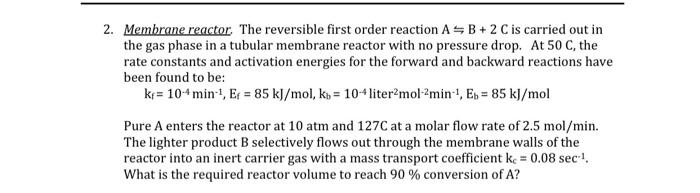
2. Membrane reactor. The reversible first order reaction ASB+ 2 C is carried out in the gas phase in a tubular membrane reactor with no pressure drop. At 50 C, the rate constants and activation energies for the forward and backward reactions have been found to be: kr = 104 min-1, Ep = 85 kJ/mol, ko = 10-4 liter molamin', E. = 85 kJ/mol Pure A enters the reactor at 10 atm and 127C at a molar flow rate of 2.5 mol/min. The lighter product B selectively flows out through the membrane walls of the reactor into an inert carrier gas with a mass transport coefficient k = 0.08 sec ! What is the required reactor volume to reach 90 % conversion of A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


