Question: Please answer the questions fully clearly showing all calculations, steps and values. Please make sure all the values are correct. Preferably hand written answers. Consider
Please answer the questions fully clearly showing all calculations, steps and values. Please make sure all the values are correct. Preferably hand written answers.
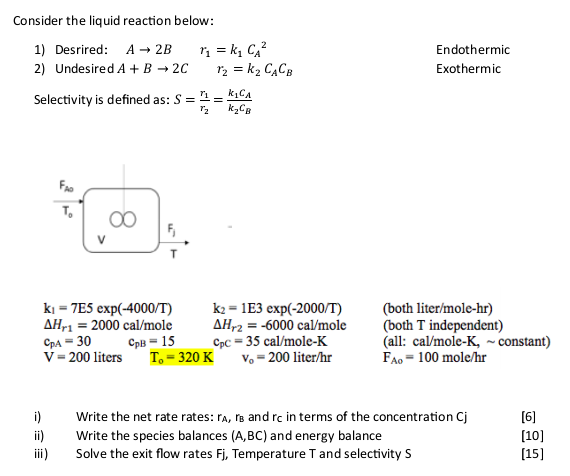
Consider the liquid reaction below: 1) Desrired: A2Br1=k1CA2 2) Undesired A+B2Cr2=k2CACB Selectivity is defined as: S=r2r1=k2cBk1cA Endothermic Exothermic k1=7E5exp(4000/T)Hr1=2000cal/molecpA=30cpB=15V=200litersk2=1E3exp(2000/T)Hr2=6000cal/molecpC=35cal/moleKT0=320Kv0=200liter/hr k2=1E3exp(2000/T) Hr2=6000cal/mole cpcc=35cal/moleK v0=200 liter /hr (both liter/mole-hr) (both T independent) (all: cal/moleK, constant) FAo=100mole/hr i) Write the net rate rates: rA,rB and rc in terms of the concentration Cj [6] ii) Write the species balances (A,BC) and energy balance [10] iii) Solve the exit flow rates Fj, Temperature T and selectivity S [15] Consider the liquid reaction below: 1) Desrired: A2Br1=k1CA2 2) Undesired A+B2Cr2=k2CACB Selectivity is defined as: S=r2r1=k2cBk1cA Endothermic Exothermic k1=7E5exp(4000/T)Hr1=2000cal/molecpA=30cpB=15V=200litersk2=1E3exp(2000/T)Hr2=6000cal/molecpC=35cal/moleKT0=320Kv0=200liter/hr k2=1E3exp(2000/T) Hr2=6000cal/mole cpcc=35cal/moleK v0=200 liter /hr (both liter/mole-hr) (both T independent) (all: cal/moleK, constant) FAo=100mole/hr i) Write the net rate rates: rA,rB and rc in terms of the concentration Cj [6] ii) Write the species balances (A,BC) and energy balance [10] iii) Solve the exit flow rates Fj, Temperature T and selectivity S [15]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


