Question: please answer them clearly and showing full solution. (8) At 1 atm, 25 mg of a protein are dissolved in 0.12 kg of pure water.
please answer them clearly and showing full solution.
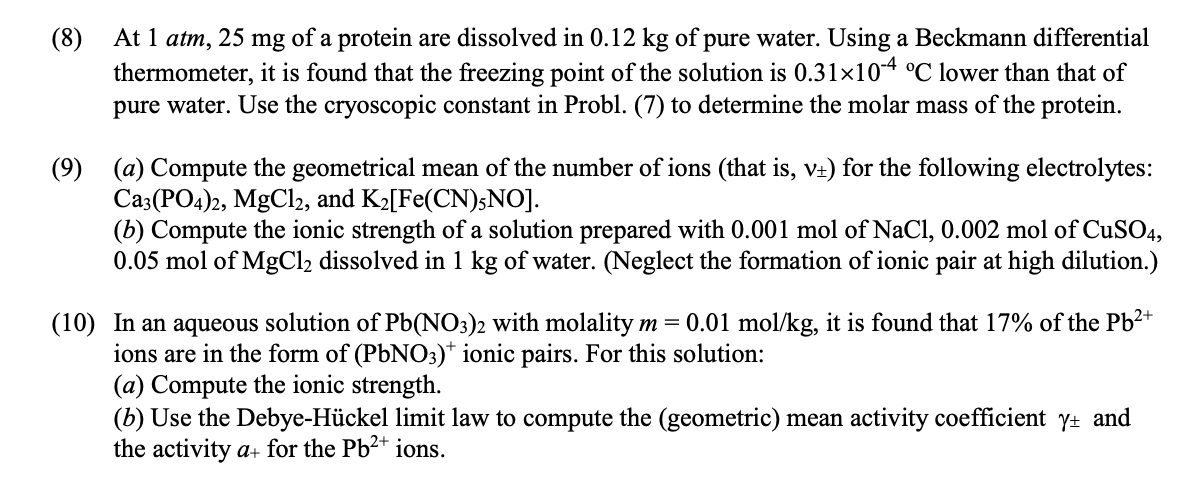
(8) At 1 atm, 25 mg of a protein are dissolved in 0.12 kg of pure water. Using a Beckmann differential thermometer, it is found that the freezing point of the solution is 0.31x10-4 C lower than that of pure water. Use the cryoscopic constant in Probl. (7) to determine the molar mass of the protein. (9) (a) Compute the geometrical mean of the number of ions (that is, v+) for the following electrolytes: Ca3(PO4)2, MgCl2, and K2[Fe(CN)5NO]. (6) Compute the ionic strength of a solution prepared with 0.001 mol of NaCl, 0.002 mol of CuSO4, 0.05 mol of MgCl2 dissolved in 1 kg of water. (Neglect the formation of ionic pair at high dilution.) (10) In an aqueous solution of Pb(NO3)2 with molality m= 0.01 mol/kg, it is found that 17% of the Pb2+ ions are in the form of (PbNO3)+ ionic pairs. For this solution: (a) Compute the ionic strength. (b) Use the Debye-Hckel limit law to compute the (geometric) mean activity coefficient yu and the activity a+ for the Pb2+ ions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


