Question: please answer them correctly and show all work 3. Consider the radial wavefunctions for the 4s and 3d orbitals. The 4s radial wavefunction is typed
please answer them correctly and show all work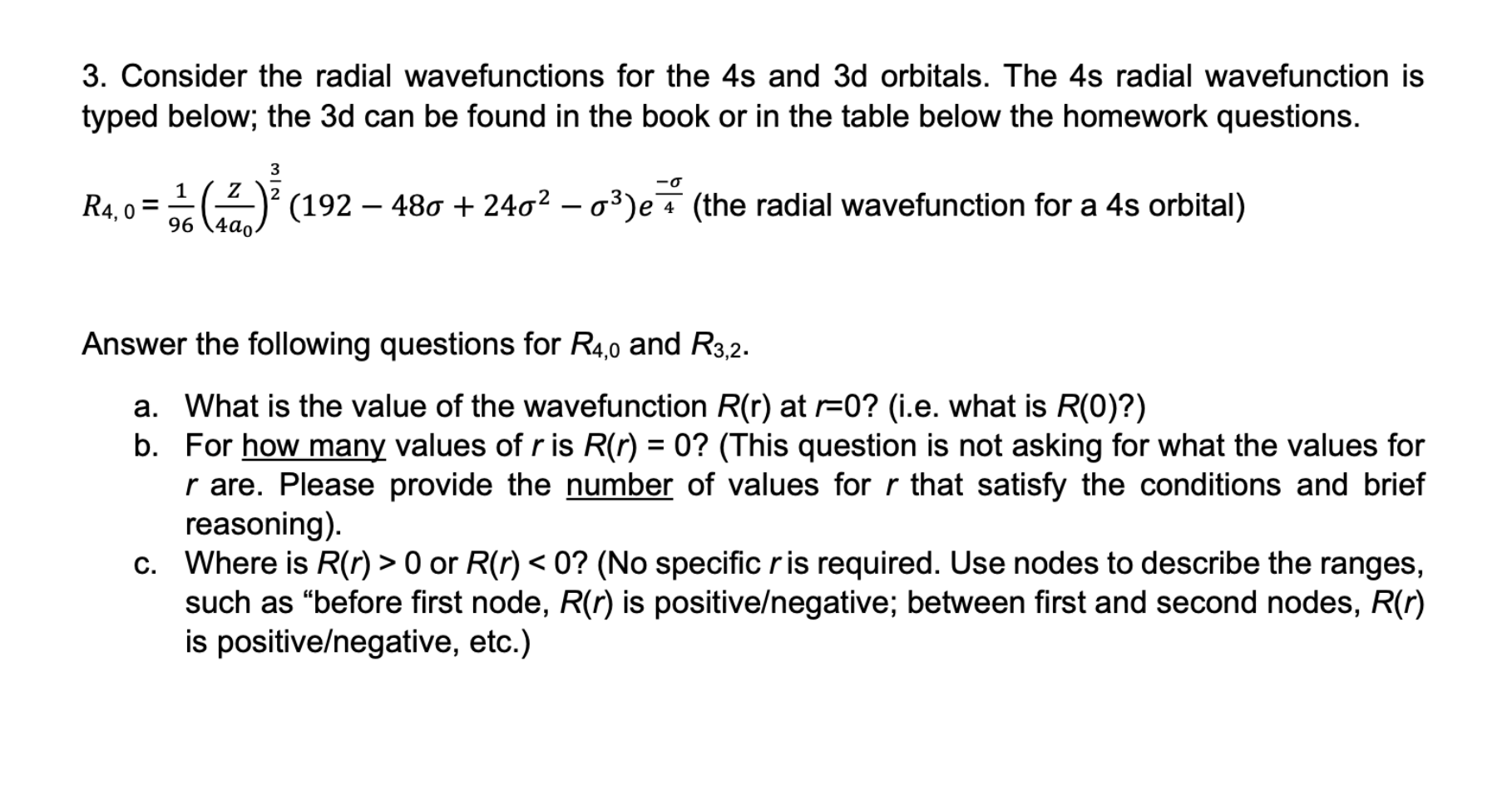
3. Consider the radial wavefunctions for the 4s and 3d orbitals. The 4s radial wavefunction is typed below; the 3d can be found in the book or in the table below the homework questions. R4,0=961(4a0Z)23(19248+2423)e4 (the radial wavefunction for a 4s orbital) Answer the following questions for R4,0 and R3,2. a. What is the value of the wavefunction R(r) at r=0 ? (i.e. what is R(0) ?) b. For how many values of r is R(r)=0 ? (This question is not asking for what the values for r are. Please provide the number of values for r that satisfy the conditions and brief reasoning). c. Where is R(r)>0 or R(r)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


