Question: A silicate mineral composed of Si, Mn and O ions Si has a coordination number of 4 Mn has a coordination number of 6
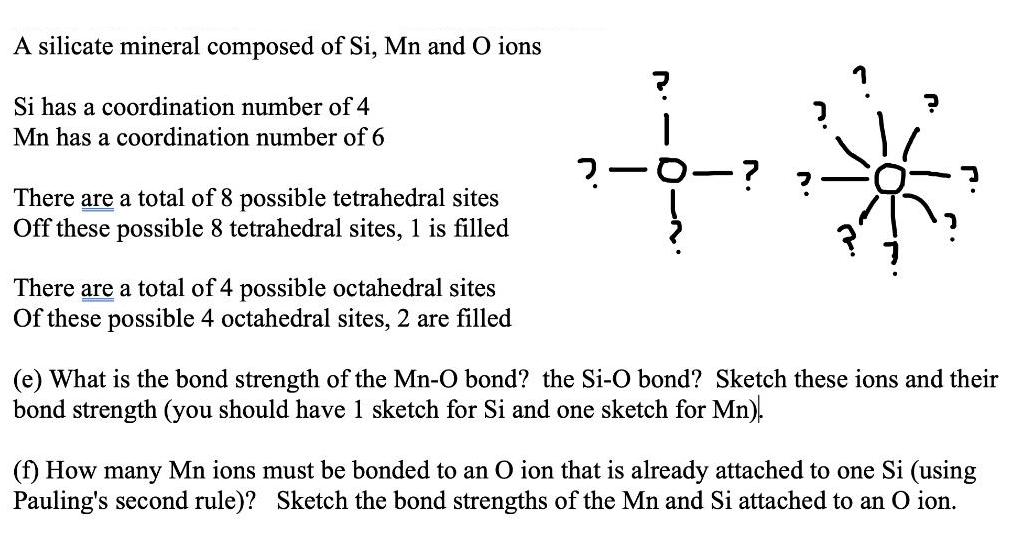
A silicate mineral composed of Si, Mn and O ions Si has a coordination number of 4 Mn has a coordination number of 6 2-0-? There are a total of 8 possible tetrahedral sites Off these possible 8 tetrahedral sites, 1 is filled There are a total of 4 possible octahedral sites Of these possible 4 octahedral sites, 2 are filled (e) What is the bond strength of the Mn-O bond? the Si-O bond? Sketch these ions and their bond strength (you should have 1 sketch for Si and one sketch for Mn). (f) How many Mn ions must be bonded to an O ion that is already attached to one Si (using Pauling's second rule)? Sketch the bond strengths of the Mn and Si attached to an O ion.
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
To address the questions well use Paulings rules which help determine bond strength based on coordin... View full answer

Get step-by-step solutions from verified subject matter experts


