Question: please answer this 3 question I need help with them. Thank you this two are one question What is the total amount of heat released
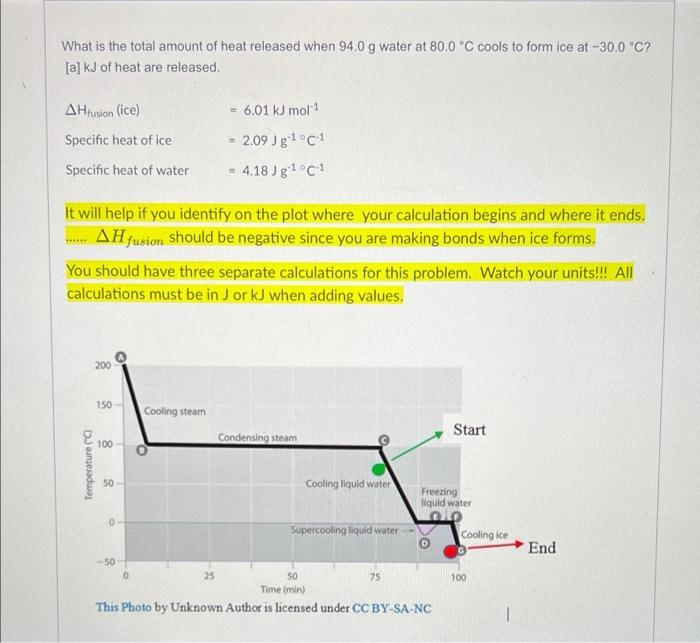


![at -30.0 C? [a] kJ of heat are released. 6.01 kJ mol-1](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f90290bf033_15266f90290639ed.jpg)
What is the total amount of heat released when 94.0 g water at 80.0C cools to form ice at -30.0 C? [a] kJ of heat are released. 6.01 kJ mol-1 A Hrusion (ice) Specific heat of ice Specific heat of water 2.09 Jg1c1 4.18 Jg1C 1 It will help if you identify on the plot where your calculation begins and where it ends. AH fusion should be negative since you are making bonds when ice forms. You should have three separate calculations for this problem. Watch your units!!! All calculations must be in Jor kJ when adding values. 200 150 Cooling steam Start 100 Condensing steam Temperature 50 Cooling liquid water Freezing liquid water 0 Supercooling liquid water Cooling Ice End 100 50 25 50 75 Time min) This Photo by Unknown Author is licensed under CC BY-SA-NC m = Practice with molality. moles of solute kg of solvent What is the molality of a 19.4 M sodium hydroxide solution that has a density of 1.54 g/mL? Consider, molality requires two components, moles of solute and kg of solvent. There are moles of solute, NaOH. No need for calculation....the numerator of Molarity = the moles of solute. From the definition of Molarity, you know the volume of solution - 1 Liter, or 1000 ml. Using the as a conversion factor, there are grams of solution. Since the denominator in Molarity includes the solute + the solvent, there are grams of solvent present. (Hint: moles of NaOH must be changed to grams of NaOH to determine the grams of solvent present). You now have both components needed to calculate the molality of the solution. The molality of the solution is m. Each of your answers should have 3 significant figures. Pre-Lab: Example Problem: Determine the molecular weight of acetic acid if a solution that contains 30.0 grams of acetic acid per kilogram of water freezes at -0.93C (you know the freezing point of pure water). Do these results agree with the assumption that acetic acid has the formula CH3COOH? Solution The freezing point depression for this solution is equal to the difference between the freezing point of the solution (-0.93C ) and the freezing point of pure water (0C). Ti = 0.93C Using the equation that defines freezing point depression, the molality can be determined: AT, = Ky x m Since we know the change in the freezing point, and K, is a constant (1.86 C/m for water): ATS m = 0.93 1.NO = 0.50 m At this point, return to the statement of the problem, to see if you are making progress toward an answer. According to this calculation, there are 0.50 moles of acetic acid per kilogram of water in this solution. The problem stated that there were 30.0 grams of acetic acid per kilogram of water in the solution. Since we simultaneously know the number of grams and the number of moles of acetic acid in this sample, we can calculate the molecular weight of acetic acid. 30.09 60. 0.50ml mol toward an answer. According to this calculation, there are 0.50 moles of acetic acid per kilogram of water in this solution. The problem stated that there were 30.0 grams of acetic acid per kilogram of water in the solution. Since we simultaneously know the number of grams and the number of moles of acetic acid in this sample, we can calculate the molecular weight of acetic acid. 30.09 0.50mol 60.-9 mol The results of this experiment are in good agreement with the molecular weight (60.05 g/mol) expected if the formula for acetic acid is CH3COOH. Now it is your turn: A solution of 2.41 grams of solute in 77.0 grams of a solvent (K4 = 8.44 for the solvent; Tfreezing of the pure solvent = 81.9 C) is found to have a freezing point of 78.6 C. What is the solute's molecular weight? (Hint: There is one difference in this problem compared to the example...the solute is dissolved in only 77.0 grams of solvent. You will need to add one step to obtain the correct answer.) _g/mol Your answer should have 3 sig figs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


