Question: please answer this question asap needed urgently will do thumbs up immediately Question 1 -- Quarter 1 (10 pts) Thursday, December 17, 2020 9:59 PM
 please answer this question asap needed urgently will do thumbs up immediately
please answer this question asap needed urgently will do thumbs up immediately
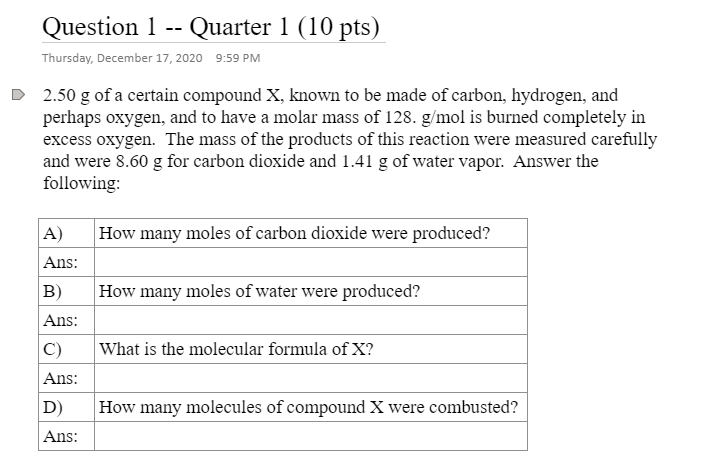
Question 1 -- Quarter 1 (10 pts) Thursday, December 17, 2020 9:59 PM 2.50 g of a certain compound X, known to be made of carbon, hydrogen, and perhaps oxygen, and to have a molar mass of 128. g/mol is burned completely in excess oxygen. The mass of the products of this reaction were measured carefully and were 8.60 g for carbon dioxide and 1.41 g of water vapor. Answer the following: A How many moles of carbon dioxide were produced? Ans: How many moles of water were produced? B) Ans: C) What is the molecular formula of X? Ans: How many molecules of compound X were combusted? D) Ans
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


