Question: Please answer this question. Pleas USE BOX METHOD as shown in the problem. Thank you! Consider the following chemical reaction. 3 Zn(CHO),(aq) + 2 Na,
Please answer this question. Pleas USE BOX METHOD as shown in the problem. Thank you!
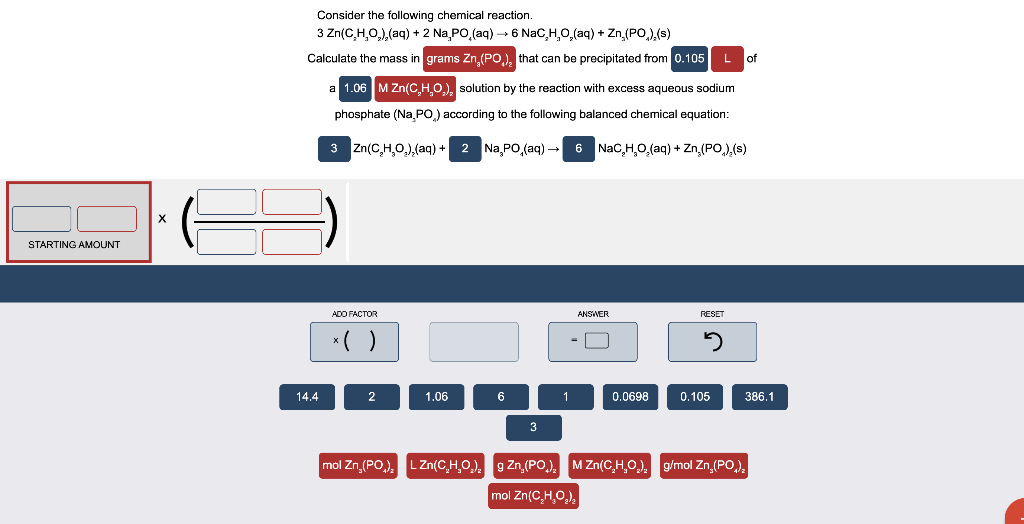
Consider the following chemical reaction. 3 Zn(CHO),(aq) + 2 Na, PO (aq) + 6 NaC,H,O, (aq) + Zn (PO),(s) Calculate the mass in grams Zn,(PO), that can be precipitated from 0.105 Lof a 1.06 M Zn(C,H,0.), solution by the reaction with excess aqueous sodium phosphate (Na PO,) according to the following balanced chemical equation: 3 Zn(C,H,O.),(aq) + 2 Na PO, (aq) 6 NaC,H,O, (aq) Zn (PO).(s) STARTING AMOUNT ADO FACTOR ANSWER RESET ( ) - 2 14.4 2 1.06 6 1 0.0698 0.105 386.1 3 mol Zn,(PO) LZn(CHO). Zn,(PO) MZ (CHO), g/mol Zn,(PO.) mol Zn(C,H,02)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


