Question: Please answer tow questions Q 11.3 11.30 Solid white phosphorus has a conventional standard Gibbs energy of zero at 25 .C. The melting point is
Please answer tow questions
Q 11.3
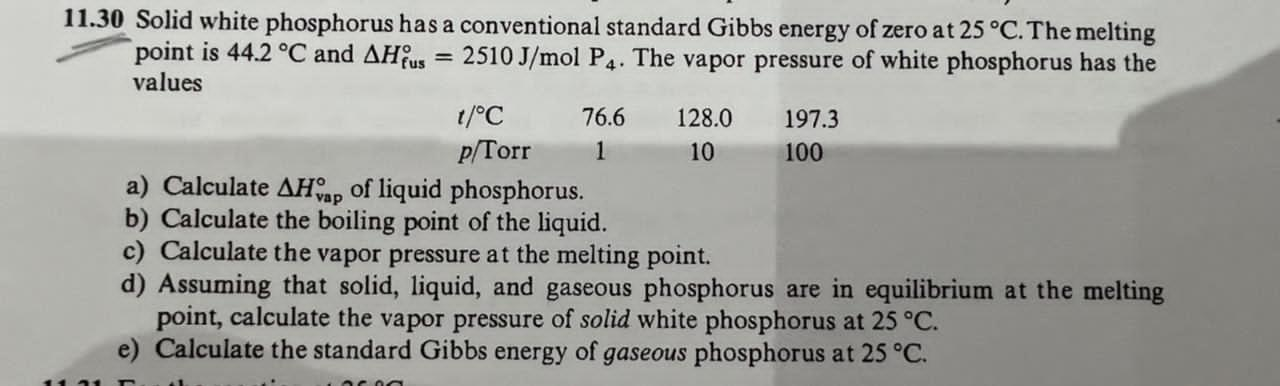

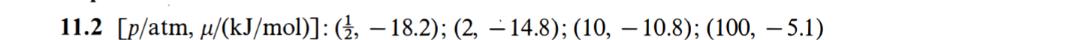
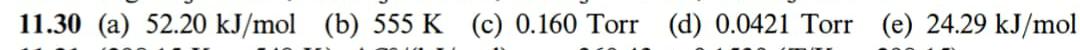
11.30 Solid white phosphorus has a conventional standard Gibbs energy of zero at 25 .C. The melting point is 44.2 C and AHus = 2510 J/mol P4. The vapor pressure of white phosphorus has the values 1/C 76.6 128.0 197.3 p/Torr 1 10 100 a) Calculate AHap of liquid phosphorus. b) Calculate the boiling point of the liquid. c) Calculate the vapor pressure at the melting point. d) Assuming that solid, liquid, and gaseous phosphorus are in equilibrium at the melting point, calculate the vapor pressure of solid white phosphorus at 25 .C. e) Calculate the standard Gibbs energy of gaseous phosphorus at 25 .C.11.2 The conventional standard Gibbs energy of ammonia at 25 .C is - 16.5 kJ/mol. Calculate the value of the molar Gibbs energy at 4, 2, 10, and 100 atm.2 [p/atm, u/(kJ/mol)]: (4, 18.2); (2, -14.8); (10, 10.8); (100, 5.1)11.30 (a) 52.20 kJ/mol (b) 555 K (c) 0.160 Torr (d) 0.0421 Torr (e) 24.29 kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


