Question: please answer Use the general equation dS=TCVdT+KdV to calculate the change in entropy of 500g of copper when it is heated from 298K to 398K
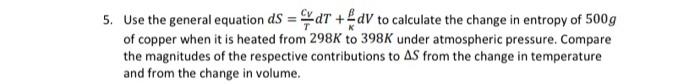
Use the general equation dS=TCVdT+KdV to calculate the change in entropy of 500g of copper when it is heated from 298K to 398K under atmospheric pressure. Compare the magnitudes of the respective contributions to S from the change in temperature and from the change in volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


