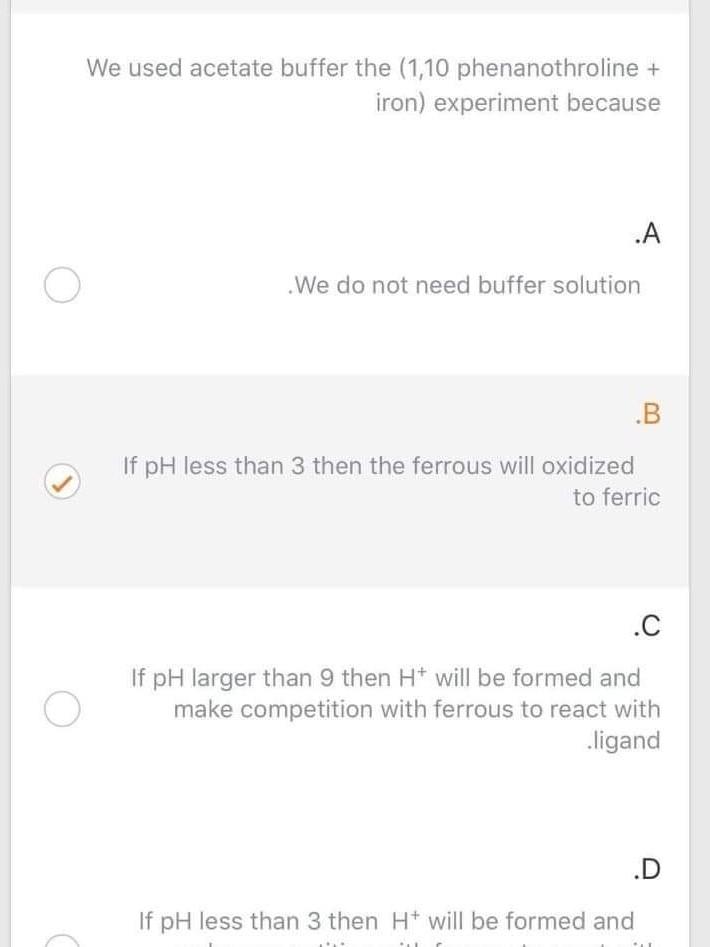
Question: Please answer We used acetate buffer the (1,10 phenanothroline + iron) experiment because .A o . We do not need buffer solution .B If pH
Please answer
We used acetate buffer the (1,10 phenanothroline + iron) experiment because .A o . We do not need buffer solution .B If pH less than 3 then the ferrous will oxidized to ferric .C If pH larger than 9 then H+ will be formed and make competition with ferrous to react with ligand If pH less than 3 then H+ will be formed and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


