Question: please answer willl rate !! A chemical reaction involves the reactants A and B, and the product C. A+BC Use the experimental data above and
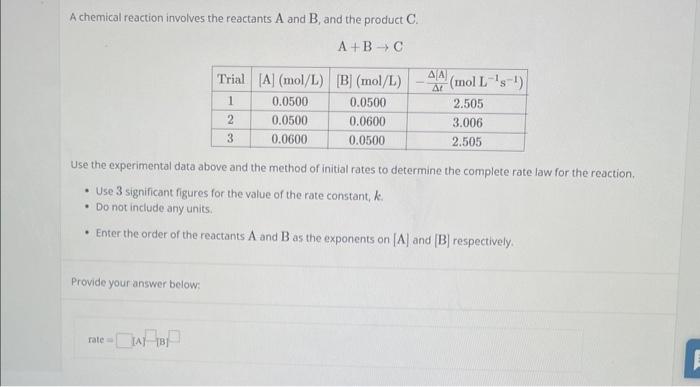
A chemical reaction involves the reactants A and B, and the product C. A+BC Use the experimental data above and the method of initial rates to determine the complete rate law for the reaction, - Use 3 significant figures for the value of the rate constant, k. - Do not include any units. - Enter the order of the reactants A and B as the exponents on [A] and [B] respectively. Provide your answer below: rate=A[B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


